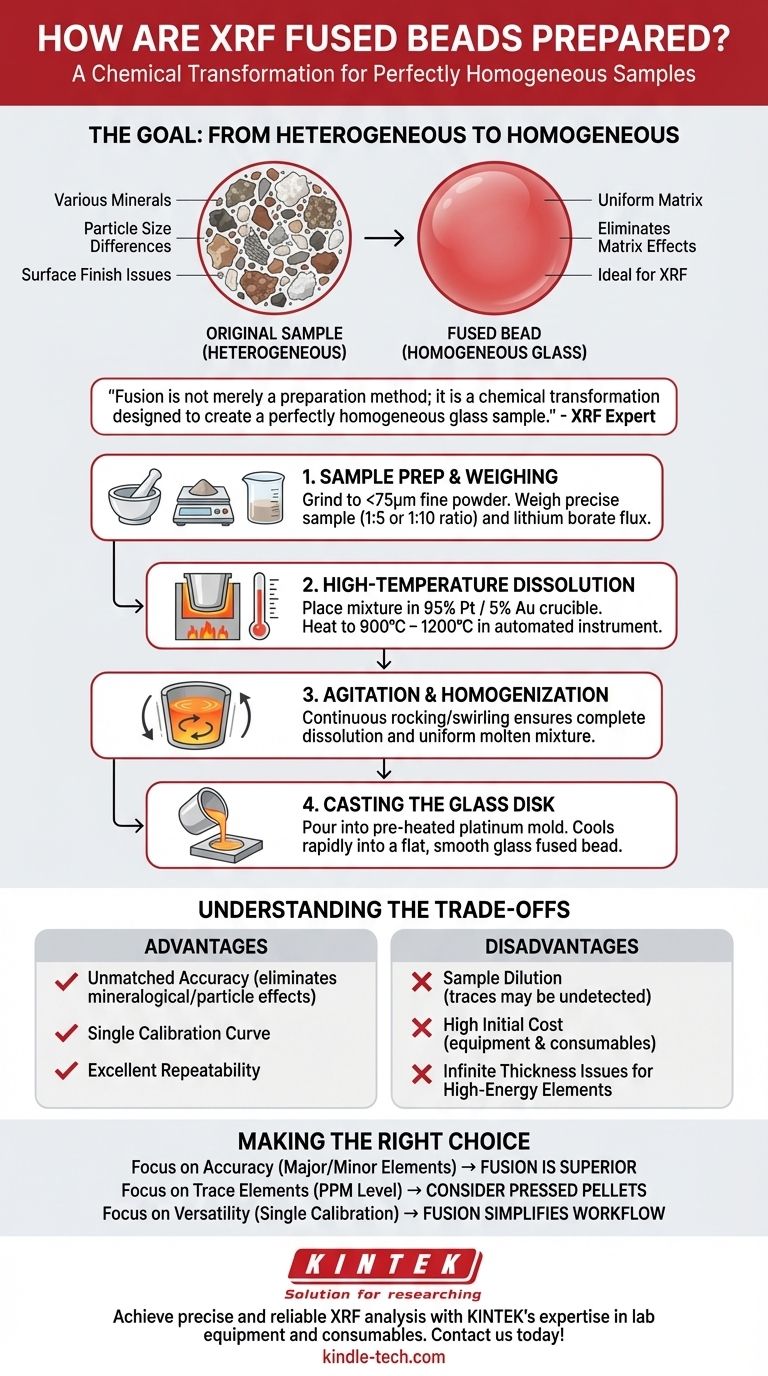
To prepare an XRF fused bead, you dissolve a finely powdered and oxidized sample in a flux (like lithium borate) at high temperatures, typically around 1000°C within a platinum crucible. This molten mixture is agitated to ensure it is perfectly homogeneous and then poured into a mold, where it cools into a uniform glass disk ready for analysis. This process effectively eliminates inconsistencies from the sample's original physical and mineralogical state.
Fusion is not merely a preparation method; it is a chemical transformation designed to create a perfectly homogeneous glass sample. This process eliminates the physical and mineralogical "matrix effects" that can compromise XRF accuracy, but it comes at the cost of diluting the sample and requiring significant initial investment.

The Goal of Fusion: From Heterogeneous to Homogeneous
The core principle behind fusion is to completely destroy the original sample's structure. Materials like rocks, cements, or ores are heterogeneous, meaning their composition and crystal structure vary from one microscopic point to another.
Why This Matters for XRF
X-ray Fluorescence (XRF) analysis is highly sensitive to these variations. Factors like particle size, mineralogy, and surface finish can scatter or absorb X-rays inconsistently, leading to inaccurate results. Fusion solves this by dissolving the sample into a new, uniform glass matrix.
The Role of the Flux
The flux, typically a lithium borate salt (like lithium tetraborate or metaborate), acts as a high-temperature solvent. It is chosen for its ability to dissolve a wide range of oxidized materials and its low absorption of the X-rays used in analysis.
The Importance of Oxidation
For the sample to dissolve completely in the flux, it must be fully oxidized. Most fusion programs include an oxidation step, or an oxidizing agent (like lithium nitrate) is added to the mix. This ensures elements are in their highest oxidation state and can properly integrate into the molten glass.
The Step-by-Step Fusion Process
While automated fusion machines handle the high-temperature steps, the operator's precision is critical for an accurate outcome.
Step 1: Sample Preparation and Weighing
The raw sample must be ground into a very fine powder (typically less than 75 microns). A precise amount of sample and flux are then weighed. The sample-to-flux ratio is a critical parameter, with common ratios being 1:5 or 1:10.
Step 2: High-Temperature Dissolution
The weighed sample and flux mixture are placed into a crucible, most commonly made of a 95% platinum / 5% gold alloy. The crucible is loaded into an automated fusion instrument which heats it to temperatures between 900°C and 1200°C.
Step 3: Agitation and Homogenization
During the heating cycle, the instrument continuously agitates the crucible by rocking or swirling it. This ensures the sample dissolves completely and the molten mixture becomes perfectly uniform.
Step 4: Casting the Glass Disk
Once homogenization is complete, the molten glass is poured into a pre-heated mold, which is also typically made of platinum. The mixture cools rapidly and solidifies into a flat, smooth glass disk (the "fused bead") that is ideal for XRF analysis.
Understanding the Trade-offs
Fusion is the gold standard for accuracy in many applications, but it is not always the best choice. Understanding its pros and cons is key.
Advantage: Unmatched Accuracy
By creating a homogeneous sample, fusion virtually eliminates mineralogical and particle-size effects. This allows for extremely accurate and repeatable analysis of major and minor elements and enables the use of a single calibration curve for many different material types.
Disadvantage: Sample Dilution
The biggest drawback is dilution. Adding a large amount of flux significantly lowers the concentration of every element in the sample. This can push trace elements (those in the parts-per-million range) below the detection limits of the XRF instrument.
Disadvantage: High Initial Cost
Fusion requires a significant investment. Automated fusion instruments, platinum crucibles and molds, and the ongoing cost of high-purity flux make it far more expensive than alternative methods like preparing pressed pellets.
Disadvantage: Infinite Thickness Issues
Fused beads are relatively thin (around 3mm). For heavy, high-energy elements (like Molybdenum or Silver), the X-rays can pass entirely through the bead. This violates the "infinitely thick" assumption required for many XRF calculations, leading to potential inaccuracies for those specific elements.
Making the Right Choice for Your Goal
Deciding between fusion and other methods depends entirely on your analytical priorities.
- If your primary focus is the highest possible accuracy for major and minor elements (e.g., in cements, ores, or geological samples): Fusion is the superior method because it removes the primary sources of analytical error.
- If your primary focus is analyzing trace elements at low concentrations (ppm-level): The dilution from fusion is a significant handicap, and you should consider using pressed powder pellets instead.
- If your primary focus is analyzing a wide variety of material types with a single calibration: Fusion provides unmatched versatility, simplifying calibration and improving lab workflow once established.
Ultimately, fusion is a powerful technique that trades sample concentration and higher costs for unparalleled accuracy and homogeneity in your results.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1. Sample Prep | Grind & weigh sample and flux | Ensure fine powder and precise ratio (e.g., 1:5 or 1:10) |
| 2. Dissolution | Heat to 1000°C in Pt crucible | Melt and dissolve sample in lithium borate flux |
| 3. Homogenization | Agitate molten mixture | Achieve perfect uniformity for accurate XRF analysis |
| 4. Casting | Pour into mold to cool | Form a flat, stable glass disk (fused bead) |
Achieve precise and reliable XRF analysis with KINTEK's expertise in lab equipment and consumables. Our fusion solutions, including high-quality platinum crucibles and fluxes, are designed to eliminate matrix effects and deliver superior accuracy for your geological, cement, or ore samples. Let our specialists help you optimize your sample preparation workflow. Contact us today to discuss your laboratory needs!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Lab Infrared Press Mold
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- What role does a laboratory hydraulic press play in preparing solid model materials? Standardize for Precise Data.
- How is a laboratory hydraulic press used for LLZTO pellets? Achieve 93% Density in Solid-State Battery Research
- How do laboratory hydraulic presses facilitate biomass pelletization? Optimize Biofuel Density and Prevent Slagging
- What is the function of a bench-top laboratory hydraulic press for XRF? Maximize Accuracy in Prosopis juliflora Analysis
- What is KBr disc method? A Complete Guide to IR Spectroscopy Sample Prep



















