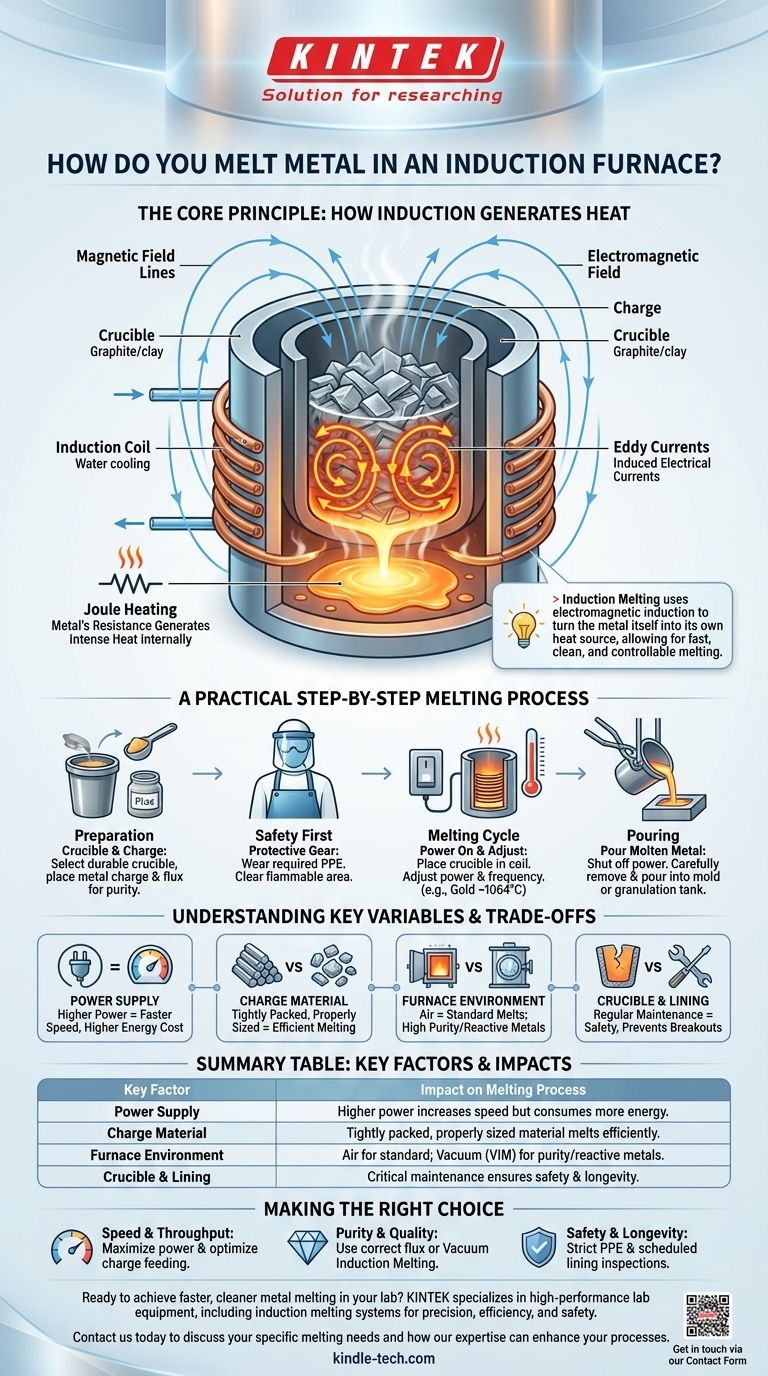
Melting metal in an induction furnace is a process of using electromagnetic fields to generate heat directly within the metal itself. Unlike a traditional furnace that uses an external flame or heating element, an induction furnace's copper coil creates a powerful, changing magnetic field. This field induces strong electrical currents, known as eddy currents, inside the metal, and the metal's natural resistance to these currents generates intense, rapid heat, causing it to melt.
The core principle of induction melting is not about applying external heat, but about using electromagnetic induction to turn the metal itself into its own heat source. This allows for extremely fast, clean, and controllable melting without direct contact between the heating element and the material.

The Core Principle: How Induction Generates Heat
To effectively operate an induction furnace, it's crucial to understand the physics at work. The process is a chain of energy conversions that happens almost instantaneously.
From Wall Power to Magnetic Field
The furnace begins by taking standard three-phase alternating current (AC) from the power grid. A power supply unit converts this low-frequency current into a high-frequency current. This high-frequency AC is then sent to the induction coil.
The Role of the Induction Coil
The induction coil, typically made of copper tubing, is the heart of the furnace. As the high-frequency current flows through it, the coil generates a powerful and rapidly alternating magnetic field in the space within the coil.
Inducing Eddy Currents in the Metal
When you place a conductive metal (the "charge") inside this magnetic field, the field lines cut through the metal. According to Faraday's law of induction, this induces powerful, swirling electrical currents within the metal, known as eddy currents.
Joule Heating: The Final Step
The metal has a natural electrical resistance. As these strong eddy currents flow against this resistance, they generate tremendous heat through a process called Joule heating. This heat is generated inside the metal, leading to rapid and uniform melting from the inside out.
A Practical Step-by-Step Melting Process
While the specific parameters vary by metal and furnace size, the operational sequence follows a clear and logical path.
Preparation: The Crucible and Charge
First, select a crucible, which is the container that holds the metal. It must withstand extreme temperatures and is typically made of graphite or clay. The metal to be melted, known as the charge, is placed inside this crucible.
Flux and Impurity Removal
To ensure a high-purity final product, a flux is often added with the charge. Flux, commonly a mixture of borax and sodium carbonate, melts and combines with impurities, forming a lighter slag that floats to the surface for easy removal.
Safety First: Essential Protective Gear
Working with molten metal is inherently hazardous. Before starting, you must wear appropriate personal protective equipment (PPE), including heat-resistant gloves, an apron, a full face shield, and safety goggles. The area must be clear of flammable materials.
The Melting Cycle
The crucible is placed inside the induction coil, and the power is turned on. You adjust the power and frequency based on the metal type and quantity. Gold, for instance, melts at approximately 1064°C. The process is fast, often taking between 2 and 20 minutes depending on the furnace's power and the size of the charge.
Pouring the Molten Metal
Once the metal is fully molten, the power is shut off. The crucible is carefully removed using tongs, and the liquid metal is poured into a mold to create an ingot, cast part, or into a granulation tank.
Understanding the Trade-offs and Key Variables
Successful induction melting is about more than just turning on the power. Several factors influence efficiency, speed, and quality.
Power vs. Speed
The most direct way to increase melting speed is to increase the power of the furnace's power supply. Higher power generates a stronger magnetic field and more intense eddy currents, but it also increases energy consumption and operational cost.
The Importance of Charge Size and Feeding
The size and type of metal you put in the furnace matter. A tightly packed charge of properly sized material will melt more efficiently than large, awkwardly shaped pieces with significant air gaps. A consistent feeding method is key to maintaining a productive cycle.
Environmental Control (Air vs. Vacuum)
Most induction furnaces operate in the open air. However, for reactive metals or alloys that require extreme purity, a vacuum induction melting (VIM) furnace is used. By melting in a vacuum, you prevent the molten metal from reacting with oxygen or nitrogen, ensuring higher quality.
Wear and Maintenance
The intense heat and chemical reactions take a toll on the furnace lining, or refractory. This lining erodes over time and requires regular inspection and repair. A damaged lining can lead to a dangerous metal breakout, so maintenance is a critical part of furnace operation.
Making the Right Choice for Your Goal
The way you operate an induction furnace should be dictated by your end goal.
- If your primary focus is speed and throughput: Maximize power output and develop an efficient process for charging the furnace with properly sized material.
- If your primary focus is metal purity and quality: Pay close attention to using the correct flux for your material, and for highly sensitive alloys, a vacuum furnace is the superior choice.
- If your primary focus is operational safety and longevity: Implement strict PPE protocols without exception and create a non-negotiable schedule for inspecting and repairing the furnace's refractory lining.
By mastering these principles, you can leverage induction technology for highly efficient, controlled, and precise metal melting.
Summary Table:
| Key Factor | Impact on Melting Process |
|---|---|
| Power Supply | Higher power increases melting speed but also energy consumption. |
| Charge Material | Tightly packed, properly sized material melts more efficiently. |
| Furnace Environment | Air for standard melts; Vacuum (VIM) for reactive metals/high purity. |
| Crucible & Lining | Regular maintenance is critical for safety and preventing breakouts. |
Ready to achieve faster, cleaner metal melting in your lab?
KINTEK specializes in high-performance lab equipment, including induction melting systems designed for precision, efficiency, and safety. Whether you're working with precious metals, alloys, or research materials, our solutions help you control purity and increase throughput.
Contact us today to discuss your specific melting needs and how our expertise can enhance your processes.
Get in touch via our Contact Form
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Lab-Scale Vacuum Induction Melting Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis
- How does a high-temperature tube furnace facilitate the phase transformation of alumina products? Master Thermal Control
- What materials are used for the tubes in tube furnaces? A Guide to Selecting the Right Tube for Your Process
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?



















