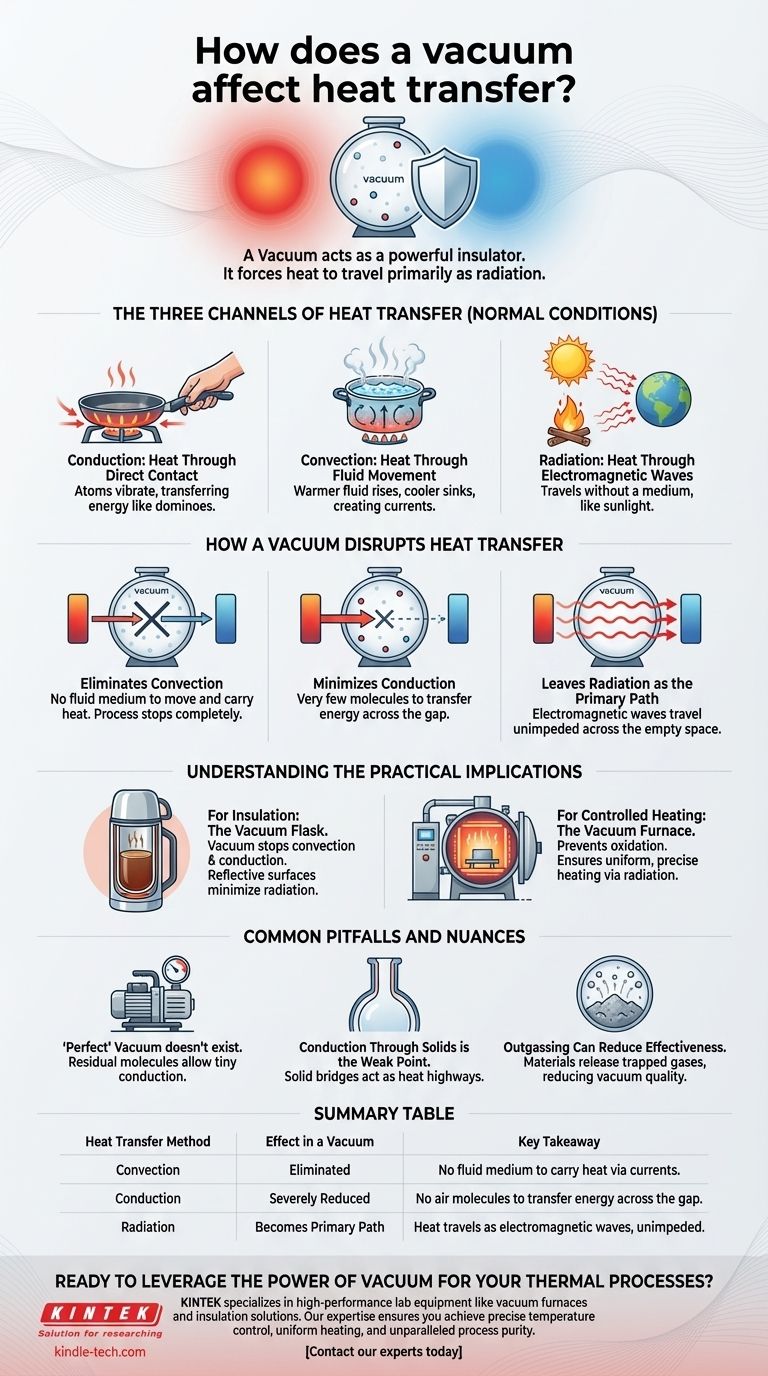
In essence, a vacuum acts as a powerful insulator by fundamentally altering how heat can travel. It virtually eliminates heat transfer by convection and drastically reduces conduction, leaving thermal radiation as the primary method for heat to move across the empty space.
A vacuum does not stop heat entirely; it changes the rules. By removing the air or other molecules that physically carry heat, it forces energy to travel as electromagnetic waves (radiation), giving you a powerful tool for either insulation or controlled heating.

The Three Channels of Heat Transfer
To understand a vacuum's effect, you must first understand the three ways heat moves from a warmer area to a cooler one.
Conduction: Heat Through Direct Contact
Conduction is the transfer of heat through direct touch. When you touch a hot pan, the heat transfers to your hand via conduction.
The atoms in the hotter material vibrate rapidly, bumping into the atoms of the cooler material and transferring their energy, like a chain reaction of falling dominoes.
Convection: Heat Through Fluid Movement
Convection is the transfer of heat through the movement of fluids (liquids or gases). This happens when a warmer, less dense fluid rises and a cooler, denser fluid sinks, creating a current.
Think of boiling water or the way a radiator heats a room. The air near the radiator gets hot, rises, and circulates, distributing the warmth.
Radiation: Heat Through Electromagnetic Waves
Radiation is heat transfer through electromagnetic waves, primarily infrared radiation. Unlike conduction and convection, it requires no medium to travel.
This is how the Sun's warmth reaches Earth across the vacuum of space or how you feel the heat from a campfire even from a distance.
How a Vacuum Disrupts Heat Transfer
A vacuum is a space devoid of matter. By removing the atoms and molecules of a gas like air, you fundamentally block two of the three heat transfer pathways.
It Eliminates Convection
This is the most significant effect. Convection is entirely dependent on the movement of a fluid medium. By removing the air from a chamber, you remove the medium.
Without a gas or liquid to form currents, convective heat transfer stops completely. There is nothing to move and carry the heat.
It Minimizes Conduction
A vacuum also severely limits conduction between objects that are not touching. While heat can still conduct through a solid object, it cannot easily conduct across an empty gap.
Without air molecules to bounce between two surfaces and carry thermal energy, this pathway is effectively shut down.
It Leaves Radiation as the Primary Path
With conduction and convection neutralized, radiation becomes the only way for heat to cross a vacuum. Any object with a temperature above absolute zero will radiate thermal energy.
In a vacuum, this radiation travels unimpeded from the hotter object to the cooler object, where it is absorbed. This is the principle behind vacuum furnaces, where glowing heating elements radiate heat onto a workpiece without any atmosphere to interfere.
Understanding the Practical Implications
This principle is exploited for two opposite goals: keeping things hot (or cold) and heating things with precision.
For Insulation: The Vacuum Flask
A thermos, or vacuum flask, is the classic example of vacuum insulation. It consists of two walls of glass or steel separated by a vacuum.
The vacuum between the walls stops heat from leaving (or entering) via convection and conduction. The surfaces are often silvered to reflect thermal radiation, tackling all three heat transfer modes and keeping your drink at its initial temperature for hours.
For Controlled Heating: The Vacuum Furnace
In industrial heat treatment, a vacuum furnace is used to heat materials to very high temperatures with extreme control.
Removing the air prevents oxidation and other chemical reactions that would occur in a normal atmosphere. More importantly, it ensures heating is uniform and predictable, as it occurs only through radiation from precisely controlled heating elements.
Common Pitfalls and Nuances
A vacuum is a powerful tool, but its limitations are important to understand.
A "Perfect" Vacuum Doesn't Exist
Real-world vacuums are simply spaces with extremely low pressure. A "high" vacuum has fewer molecules than a "low" vacuum, making it a better insulator. However, there will always be some residual molecules that allow for a tiny amount of conduction.
Conduction Through Solids is the Weak Point
A vacuum cannot stop heat from conducting through solid materials. In a vacuum flask, the only significant point of heat loss is the neck, where the inner and outer walls connect, creating a solid bridge for conduction to occur.
Outgassing Can Reduce Effectiveness
When materials are placed in a vacuum, they can release trapped gases from their surface or interior, a process called outgassing. This can slightly increase the pressure inside the chamber, reducing the vacuum's insulating effectiveness over time.
Making the Right Choice for Your Goal
To apply this knowledge, focus on which heat transfer mechanisms you need to block or utilize.
- If your primary focus is thermal insulation: Use a vacuum to create a barrier that eliminates convective and conductive heat transfer between surfaces, and use reflective coatings to minimize radiation.
- If your primary focus is controlled, uniform heating: Use a vacuum to remove interfering atmospheric gases, allowing for pure and even radiative heating without the risk of surface contamination.
- If you are designing a system: Remember that any solid material bridging the vacuum gap will act as a highway for conductive heat transfer and is often the weakest link in your insulation.
By understanding that a vacuum selectively blocks conduction and convection, you gain precise control over how heat behaves in your system.
Summary Table:
| Heat Transfer Method | Effect in a Vacuum | Key Takeaway |
|---|---|---|
| Convection | Eliminated | No fluid medium to carry heat via currents. |
| Conduction | Severely Reduced | No air molecules to transfer energy across the gap. |
| Radiation | Becomes Primary Path | Heat travels as electromagnetic waves, unimpeded. |
Ready to leverage the power of vacuum for your thermal processes? KINTEK specializes in high-performance lab equipment like vacuum furnaces and insulation solutions. Our expertise ensures you achieve precise temperature control, uniform heating, and unparalleled process purity.
Contact our experts today to discuss how our vacuum technology can enhance your laboratory's efficiency and results.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- Why are biopalladium samples processed in a vacuum drying oven? Ensuring Sample Integrity for SEM Analysis
- What are the 3 phases of quenching process? Master the Cooling Stages for Perfect Hardness
- How does sintering temperature affect density? Optimize Your Process for Maximum Material Performance
- What is the difference between sintering and smelting? Consolidation vs. Extraction Explained
- What is the capacity of a furnace? From Home Heating to Industrial Processes
- What is the vacuum heat treatment cycle? Achieve Superior Material Purity and Precision
- What changes in the annealing process? A Guide to the 3 Key Microstructural Stages
- Where is sinter used? A Guide to Its Critical Role in Manufacturing



















