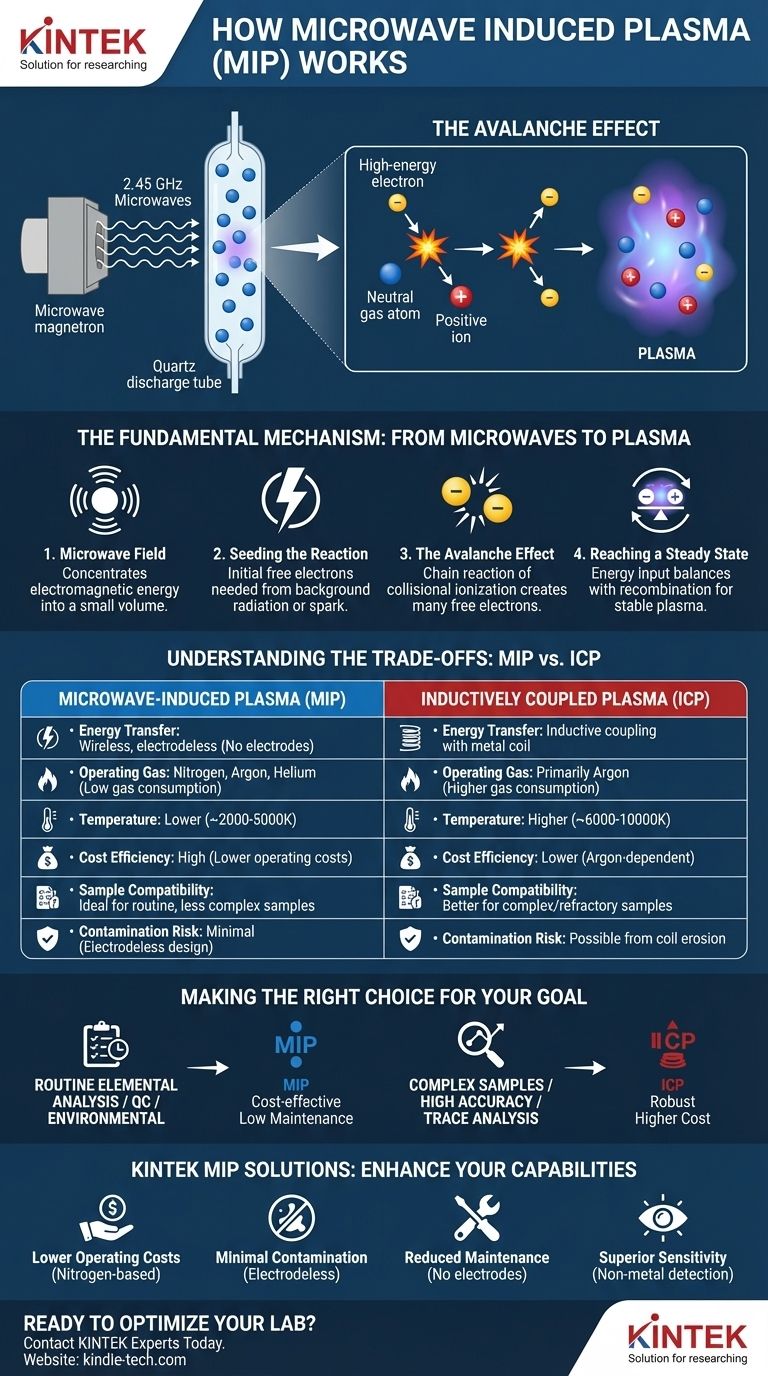
At its core, microwave-induced plasma (MIP) is a method for creating a superheated, electrically conductive gas using focused microwave energy. Similar to how a microwave oven heats food, an MIP system directs high-frequency electromagnetic waves into a chamber containing a gas. This energy strips electrons from the gas atoms, initiating a self-sustaining chain reaction that transforms the neutral gas into an intensely hot and luminous plasma.
While the physics involves complex electromagnetic interactions, the essential principle is simple: MIP uses wireless energy transfer to create a clean, electrodeless plasma. This core feature makes it uniquely suited for applications where sample purity and low operational costs are paramount.

The Fundamental Mechanism: From Microwaves to Plasma
To truly grasp how MIP works, we must look at the process step-by-step, from the initial energy input to the creation of a stable plasma.
The Role of the Microwave Field
The process begins with a microwave generator, typically a magnetron operating at 2.45 GHz. This creates a powerful, rapidly oscillating electric field that is channeled through a waveguide.
The purpose of the waveguide is to concentrate this electromagnetic energy into a very small volume, usually inside a quartz discharge tube through which a gas, like argon or nitrogen, is flowing.
Seeding the Reaction: The First Electron
A plasma cannot form without an initial "seed" charge. A few free electrons are always present in any gas due to natural background radiation.
Alternatively, a system can use a brief, high-voltage spark (from a device like a Tesla coil) to generate the first few free electrons needed to kickstart the process.
The Avalanche Effect: Collisional Ionization
Once a free electron is present in the focused, high-frequency electric field, it is rapidly accelerated back and forth.
This high-energy electron collides with a neutral gas atom. If the electron has enough kinetic energy, the collision is inelastic, knocking another electron off the atom.
This creates a positive ion and a second free electron. Now there are two electrons to be accelerated by the field, which then go on to ionize two more atoms, creating four electrons, and so on. This chain reaction is known as an electron avalanche or ionization cascade.
Reaching a Steady State
This avalanche process happens almost instantaneously, rapidly converting a portion of the gas into a mixture of free electrons, positive ions, and neutral atoms—the state of matter known as plasma.
The plasma is sustained because the microwave field continuously pumps energy into the electrons, which then transfer that energy to the heavier particles (ions and atoms) through collisions, keeping the plasma hot and ionized. The rate of ionization becomes balanced by the rate at which electrons and ions recombine, creating a stable, steady-state plasma.
Understanding the Trade-offs: MIP vs. Other Plasmas
MIP is not the only method for generating analytical plasmas. Its primary competitor is Inductively Coupled Plasma (ICP). Understanding their differences is key to choosing the right tool.
Advantage: Electrodeless Design
The most significant advantage of MIP is its electrodeless nature. The energy is coupled into the gas wirelessly.
This means there are no metal electrodes in contact with the hot plasma that can erode, wear out, or contaminate the sample. This leads to lower maintenance, longer component life, and cleaner analytical signals.
Advantage: Lower Operating Costs
MIP systems, particularly those that can run on nitrogen generated from the air, have significantly lower gas consumption and cost compared to argon-hungry ICP systems. This makes the total cost of ownership much more attractive for routine analysis.
Limitation: Lower Temperature and Robustness
An MIP is generally not as hot or as robust as an ICP. Its plasma temperature is lower, meaning it is less effective at breaking down very complex or refractory samples.
This also makes it more susceptible to matrix effects, where the presence of high concentrations of other elements in a sample can interfere with the measurement of the target element. An ICP is more resilient to these interferences.
Limitation: Analytical Sensitivity
While highly capable, MIP generally cannot achieve the same ultra-low detection limits for some elements as a modern ICP system. For trace and ultra-trace analysis, ICP often remains the superior choice.
Making the Right Choice for Your Goal
Selecting a plasma source requires aligning the technology's strengths with your specific analytical or industrial objective.
- If your primary focus is routine elemental analysis with lower sample complexity: MIP offers a cost-effective, low-maintenance, and highly capable solution, especially for environmental monitoring or quality control.
- If your primary focus is analyzing complex, varied, or difficult-to-digest samples with the highest accuracy: An Inductively Coupled Plasma (ICP) source is likely a more robust and reliable choice, despite its higher operating cost.
- If your primary focus is gas-phase analysis or detection for chromatography: MIP is an exceptional detector due to its high sensitivity to non-metals and its ability to operate with helium or nitrogen carrier gas.
Ultimately, understanding the core mechanism of MIP empowers you to leverage its unique advantages for specific and well-suited applications.
Summary Table:
| Feature | Microwave-Induced Plasma (MIP) | Inductively Coupled Plasma (ICP) |
|---|---|---|
| Energy Transfer | Wireless, electrodeless | Inductive coupling with metal coil |
| Operating Gas | Nitrogen, Argon, Helium | Primarily Argon |
| Temperature | Lower (~2000-5000K) | Higher (~6000-10000K) |
| Maintenance | Low (no electrode erosion) | Higher (coil replacement) |
| Cost Efficiency | High (lower gas consumption) | Lower (argon-dependent) |
| Sample Compatibility | Ideal for routine, less complex samples | Better for complex/refractory samples |
| Contamination Risk | Minimal (electrodeless design) | Possible from coil erosion |
Ready to enhance your laboratory's analytical capabilities?
KINTEK's microwave-induced plasma systems deliver the clean, cost-effective performance your lab needs for routine elemental analysis and gas chromatography detection. Our MIP technology provides:
• Lower operating costs with nitrogen-based operation • Minimal contamination through electrodeless design • Reduced maintenance with no consumable electrodes • Superior sensitivity for non-metal detection
Whether you're in environmental monitoring, quality control, or chromatography, KINTEK has the specialized lab equipment to optimize your workflow. Contact our experts today to discuss how MIP technology can solve your specific analytical challenges!
Visual Guide
Related Products
- Three-dimensional electromagnetic sieving instrument
- Proton Exchange Membrane for Batteries Lab Applications
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Lab-Scale Vacuum Induction Melting Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
People Also Ask
- What is the process of MPCVD? Grow High-Purity Diamond & Advanced Films
- How does microwave plasma work? Unlock Precision Material Synthesis for Advanced Manufacturing
- What is MPCVD? Unlock Atom-by-Atom Precision for High-Purity Materials
- What's the difference between CVD and HPHT? Choosing the Right Lab-Grown Diamond Method
- What are the properties of a diamond? Unlocking Hardness, Brilliance & Thermal Conductivity
- What is better lab grown or natural diamonds? A Guide to Choosing Your Perfect Stone
- What is MPCVD method? A Guide to High-Purity Diamond Synthesis
- Can a diamond be created in a laboratory? The Science Behind Genuine Lab-Grown Diamonds








