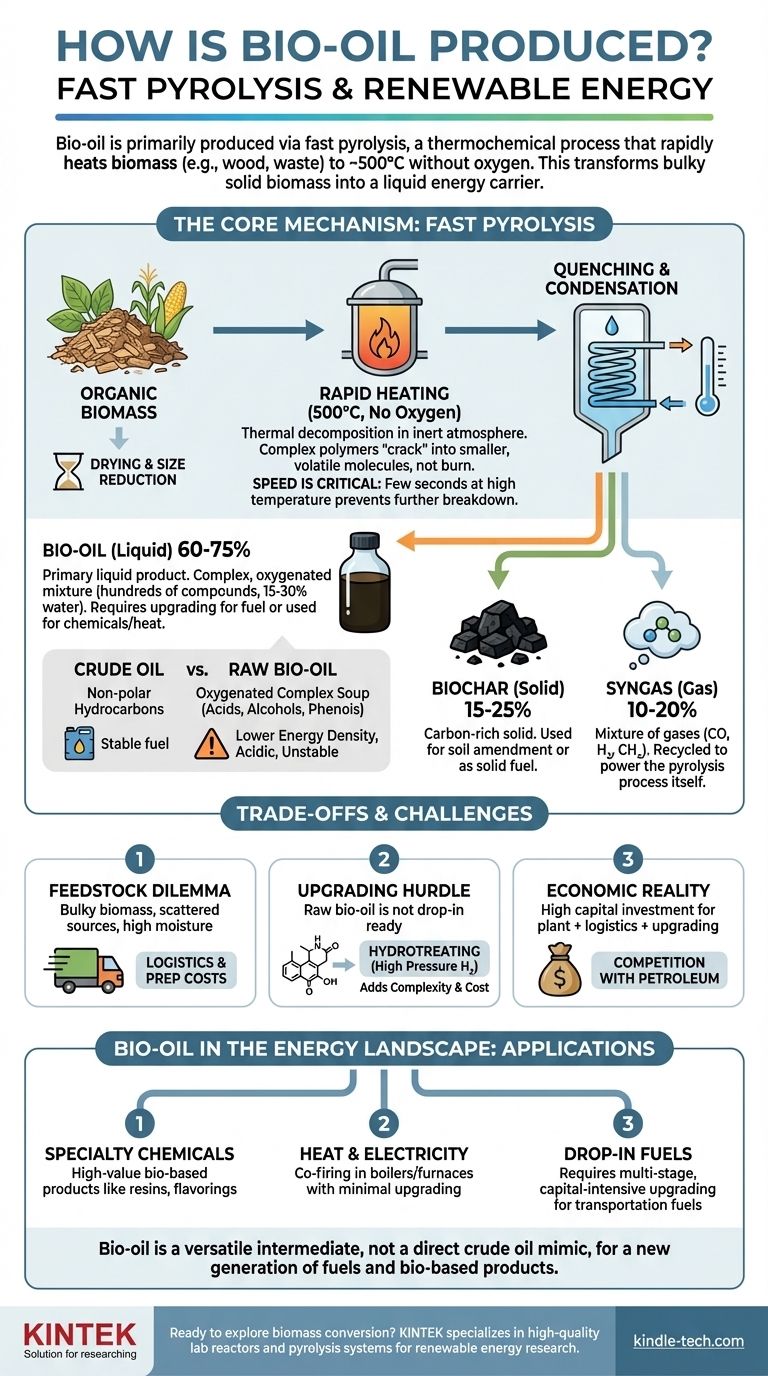
Bio-oil is produced primarily through a process called fast pyrolysis. This thermochemical technique rapidly heats organic biomass—such as wood, agricultural waste, or algae—to around 500°C in an environment with no oxygen. This intense, oxygen-starved heating prevents the biomass from combusting and instead vaporizes it, with the vapors then rapidly cooled and condensed into a dark, liquid "bio-oil."
The core purpose of bio-oil production is to transform bulky, low-density solid biomass into a liquid energy carrier that is far easier to store and transport. However, this raw bio-oil is a chemically complex, acidic, and unstable substance that requires significant upgrading to be used as a direct replacement for conventional fossil fuels.

The Core Mechanism: Understanding Fast Pyrolysis
Fast pyrolysis is an engineered process designed to maximize the liquid fuel yield from raw biomass. It operates on a delicate balance of temperature, heating rate, and time.
The Role of Extreme Heat
Pyrolysis is the thermal decomposition of materials at elevated temperatures in an inert atmosphere. It is fundamentally different from combustion because the absence of oxygen prevents the biomass from simply burning.
Instead of burning, the complex organic polymers in the biomass (like cellulose and lignin) are "cracked" into smaller, volatile molecules.
Speed is a Critical Factor
To produce bio-oil, the process must be fast pyrolysis. This means the biomass is heated extremely quickly, and the resulting vapors spend only a few seconds at high temperatures before being quenched.
This short vapor residence time is crucial. It prevents the vapors from further breaking down into non-condensable gases, thereby maximizing the yield of the desired liquid bio-oil.
The Three Primary Products
The fast pyrolysis process does not just create bio-oil. It invariably yields three distinct products that must be managed.
- Bio-oil (The Liquid): Typically makes up 60-75% of the product mass. This is the primary target for creating liquid fuels and chemicals.
- Biochar (The Solid): A carbon-rich solid similar to charcoal, making up 15-25% of the mass. It can be used as a solid fuel or as a soil amendment.
- Syngas (The Gas): A mix of non-condensable gases like carbon monoxide, hydrogen, and methane, making up 10-20% of the mass. This gas is typically recycled to provide the energy needed to heat the pyrolysis reactor itself, making the process more self-sustaining.
The Nature of Raw Bio-Oil
It is a common misconception to think of bio-oil as a direct equivalent to crude petroleum. Its chemical composition and properties are fundamentally different, presenting both opportunities and significant challenges.
A Complex Chemical Soup
Unlike crude oil, which is a mix of non-polar hydrocarbons, bio-oil is a highly oxygenated and complex mixture. It contains hundreds of different organic compounds, including acids, alcohols, aldehydes, ketones, and phenols.
A significant portion of bio-oil is also water—typically 15-30%—which is produced during the pyrolysis reaction and becomes intimately mixed with the organic compounds.
Key Properties and Implications
The high oxygen and water content give bio-oil undesirable properties. It has a lower energy density than fossil fuels, is highly acidic and corrosive to standard pipes and engines, and is chemically unstable.
Over time, raw bio-oil can thicken and even separate into different phases, a process known as aging. This makes long-term storage and use in conventional engines or refineries impossible without further processing.
Understanding the Trade-offs and Challenges
While converting solid waste into a liquid fuel is an elegant concept, the practical and economic hurdles are substantial. Success in this field requires acknowledging and addressing these core challenges.
The Feedstock Dilemma
The logistics of biomass are a major constraint. While sources like corn stover or forest residue are abundant, they are also bulky, geographically dispersed, and have high moisture content.
The cost and energy required to collect, dry, and transport this low-density feedstock to a centralized pyrolysis plant can make the final bio-oil economically uncompetitive.
The Upgrading Hurdle
Raw bio-oil cannot be used as a "drop-in" fuel. To make it compatible with existing infrastructure, it must undergo significant and costly upgrading.
The most common upgrading process is hydrotreating, which uses a catalyst and high-pressure hydrogen to remove oxygen and stabilize the molecules. This step adds significant complexity and cost to the overall fuel production chain.
The Economic Reality
Building and operating a fast pyrolysis plant requires significant capital investment. When combined with the costs of feedstock logistics and mandatory upgrading, producing bio-fuels that can compete with the price of petroleum remains a major economic challenge.
How Bio-Oil Fits into the Energy Landscape
The optimal use of bio-oil depends entirely on the end goal. It is not a one-size-fits-all solution but a platform technology with different applications.
- If your primary focus is producing specialty chemicals: Bio-oil is a promising source for extracting high-value, bio-based chemicals like phenols for resins or flavoring compounds, potentially offering a more direct path to profitability than fuel.
- If your primary focus is generating heat or electricity: Raw bio-oil can be co-fired in industrial boilers or furnaces, offering a way to displace fossil fuels like heating oil or natural gas with minimal upgrading.
- If your primary focus is creating drop-in transportation fuels: Be prepared for a capital-intensive, multi-stage process. The core technology is viable but requires integrated upgrading to produce a stable, refinery-ready intermediate.
Ultimately, understanding the production process reveals that bio-oil's true potential lies not in being a simple crude oil mimic, but a versatile intermediate for a new generation of fuels and bio-based products.
Summary Table:
| Fast Pyrolysis Product | Typical Yield (wt%) | Primary Characteristics & Uses |
|---|---|---|
| Bio-oil (Liquid) | 60-75% | Complex, oxygenated liquid; requires upgrading for fuel or can be used for heat/chemicals. |
| Biochar (Solid) | 15-25% | Carbon-rich solid; used as a soil amendment or solid fuel. |
| Syngas (Gas) | 10-20% | Mixture of gases (CO, H₂); often used to power the pyrolysis process itself. |
Ready to explore biomass conversion in your lab? The production and analysis of bio-oil require precise thermal processing equipment. KINTEK specializes in high-quality lab reactors, pyrolysis systems, and analytical tools that help researchers and engineers optimize renewable energy processes. Contact our experts today to find the right equipment for your biomass and bio-oil projects and accelerate your renewable energy innovations.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Automatic Lab Cold Isostatic Press CIP Machine Cold Isostatic Pressing
People Also Ask
- What are the end products of the plastic pyrolysis? Transform Waste into Valuable Fuels & Chemicals
- What is the purpose of a rotary furnace? Achieve Unmatched Heating Uniformity for Powders & Parts
- What are the uses of calcination process? A Guide to Material Transformation
- What is bio-oil from biomass? A Guide to Pyrolysis Oil Production and Uses
- What are the different types of pyrolysis technology? Choose the Right Process for Your Output Goal
- What waste is suitable for pyrolysis? Unlock Value from Plastics, Biomass, and Tires
- What is the process of a plastic pyrolysis plant? A Complete Guide to Converting Waste Plastic into Fuel
- Does pyrolysis produce gas? Unlocking the Potential of Syngas, Bio-oil, and Biochar



















