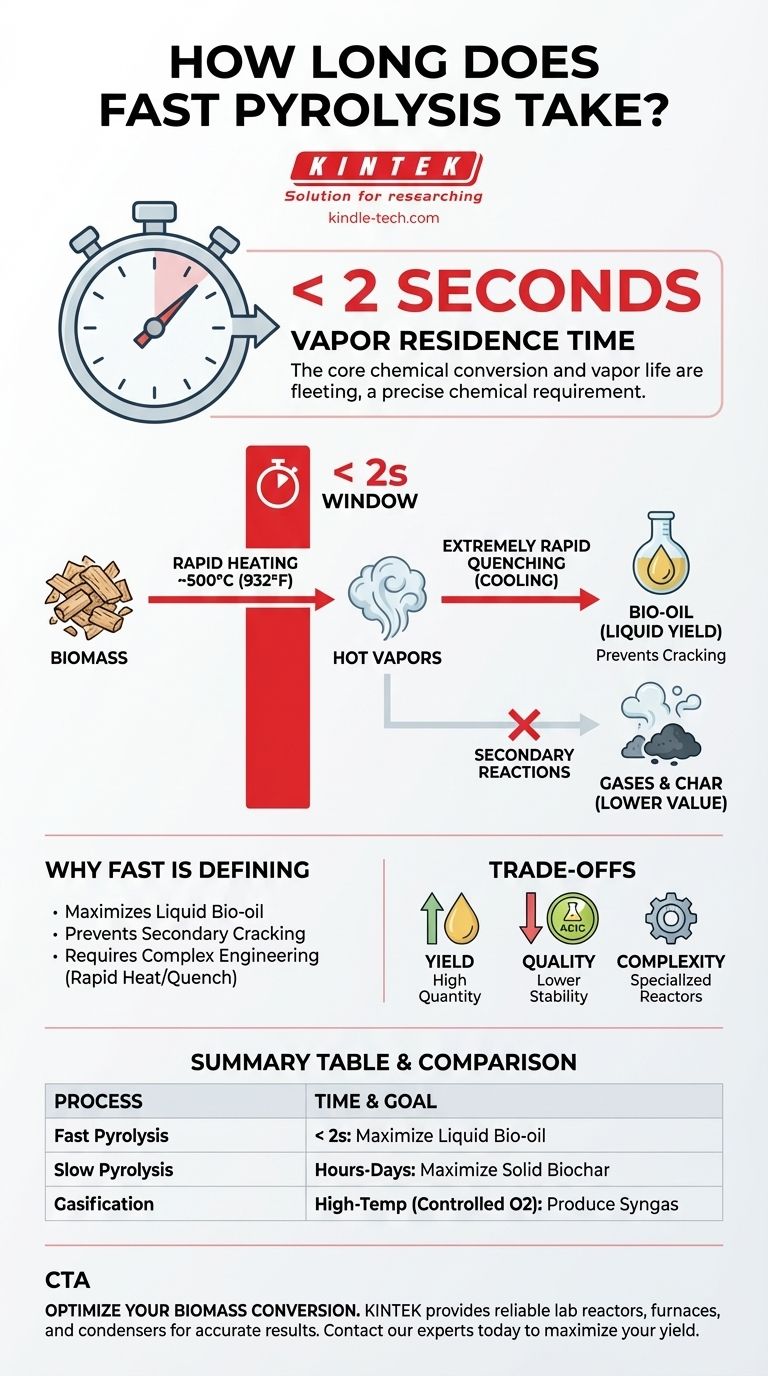
In fast pyrolysis, the core chemical conversion is over in less than two seconds. This process involves heating biomass to high temperatures in the absence of oxygen, a step which must be followed by extremely rapid cooling. The term "fast" refers specifically to this very short "vapor residence time"—the brief window in which the biomass vapors exist before they are condensed into liquid bio-oil.
The critical insight is that the extreme speed of fast pyrolysis is not for industrial throughput, but is a precise chemical requirement. This brief reaction time is essential to maximize the yield of liquid bio-oil by preventing it from breaking down further into less valuable gases.

Why "Fast" is the Defining Characteristic
The entire fast pyrolysis process is engineered around a specific time constraint. This constraint is the key to controlling the chemical output and achieving the desired liquid product.
The Goal: Maximizing Liquid Bio-oil
The primary objective of fast pyrolysis is to convert solid biomass into a high yield of a liquid product, known as bio-oil or pyrolysis oil. As the references note, this bio-oil holds potential as a renewable starting material for fuels and chemicals.
The Mechanism: Rapid Heating and Quenching
To achieve this, finely ground biomass particles are heated to around 500°C (932°F) at an extremely high rate. This thermal shock breaks down the complex polymers in the biomass (like cellulose and lignin) into smaller, volatile molecules that form a hot vapor.
Crucially, these hot vapors must be cooled, or quenched, just as rapidly. This locks in their chemical structure and condenses them into the liquid bio-oil product.
The Critical Time Window: Vapor Residence Time
The duration between the creation of the hot vapors and their quenching is the vapor residence time. This is the specific time the user's question addresses.
For fast pyrolysis to be successful, this time must be incredibly short—typically less than two seconds. This brief window is the defining feature of the entire process.
Understanding the Trade-offs of Speed
While essential for maximizing liquid yield, this speed creates significant engineering challenges and defines the quality of the resulting product.
Yield vs. Product Quality
The rapid quenching that maximizes bio-oil yield also traps many undesirable compounds in the liquid. The resulting bio-oil is highly oxygenated, acidic, and thermally unstable.
It cannot be used as a drop-in replacement for crude oil without significant and costly upgrading. The process prioritizes quantity of liquid over the quality of that liquid.
The Enemy of Yield: Secondary Reactions
The reason the vapor residence time must be so short is to prevent secondary reactions. If the hot vapors remain at high temperatures for more than a few seconds, they begin to "crack."
This secondary cracking breaks the valuable vapor molecules down further into non-condensable gases (like CO, CO₂) and solid char, drastically reducing the final liquid bio-oil yield.
Complex Engineering Requirements
Achieving heat transfer rates high enough to pyrolyze biomass in seconds is a major engineering challenge. It requires very small particle sizes and specialized reactors, such as circulating fluidized bed or ablative reactors, which add complexity and cost to the operation.
Making the Right Choice for Your Goal
The reaction time is the most critical variable in determining the output of a thermal biomass conversion process. Your intended product dictates the necessary speed.
- If your primary focus is maximizing liquid bio-oil for fuel precursors: Fast pyrolysis, with its sub-2-second vapor residence time, is the correct pathway.
- If your primary focus is producing solid biochar for soil amendment: A much slower process, slow pyrolysis (or carbonization), with residence times of hours to days, is required.
- If your primary focus is producing syngas for power or chemical synthesis: Gasification, a high-temperature process with a controlled amount of oxygen, is the appropriate technology.
Ultimately, controlling the reaction time is the fundamental lever for determining the final product you will get from biomass.
Summary Table:
| Process Aspect | Key Detail |
|---|---|
| Core Reaction Time | < 2 seconds (vapor residence time) |
| Primary Goal | Maximize liquid bio-oil yield |
| Typical Temperature | ~500°C (932°F) |
| Key Constraint | Prevent secondary cracking of vapors |
| Alternative Process (Slow Pyrolysis) | Hours to days (for biochar production) |
Ready to Optimize Your Biomass Conversion Process?
Understanding the precise reaction kinetics is crucial for selecting the right lab equipment. Whether you're researching fast pyrolysis for bio-oil, slow pyrolysis for biochar, or gasification for syngas, KINTEK provides the reliable, high-performance lab reactors, furnaces, and condensers you need to achieve accurate and repeatable results.
Contact our experts today to discuss your specific application and discover how KINTEK's specialized lab equipment can help you maximize yield and efficiency in your thermal conversion research.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the typical heating zone configurations and maximum temperature capabilities of tube furnaces? Find the Right Setup for Your Lab
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance



















