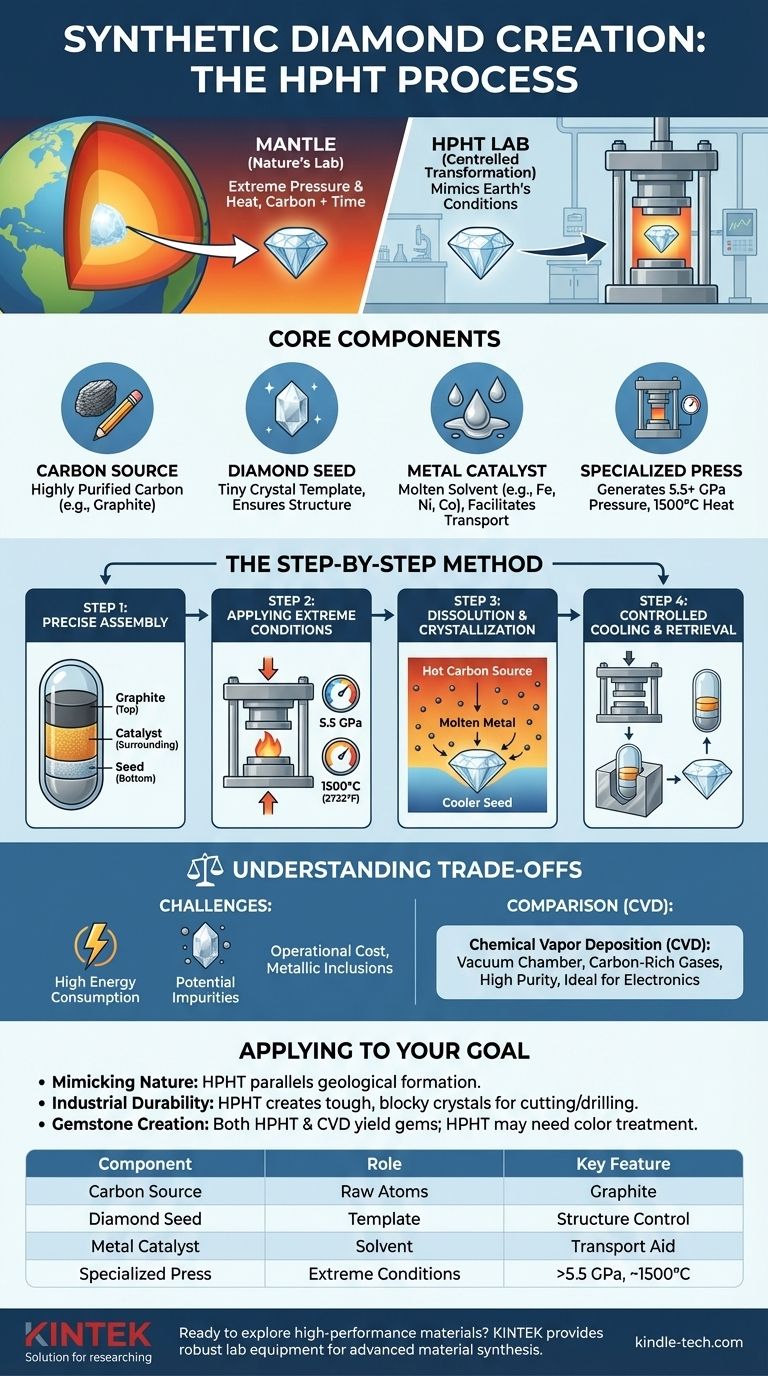
To create a synthetic diamond using pressure and temperature, scientists use a method called High-Pressure/High-Temperature (HPHT). This process precisely mimics the diamond-forming conditions deep within the Earth's mantle by subjecting a carbon source to immense pressure and heat. Inside a specialized press, this forces the carbon atoms to restructure themselves into the incredibly strong and stable crystalline lattice of a diamond.
The core principle of the HPHT method is not brute force, but controlled transformation. It uses extreme pressure and heat to dissolve a simple carbon source into a molten metal catalyst, which then allows the carbon to controllably recrystallize onto a tiny diamond seed, growing a new, larger diamond layer by layer.

The Core Components of the HPHT Process
To understand how HPHT works, you must first understand its essential ingredients. Each component plays a critical role in successfully transforming a basic element into a high-performance material.
The Carbon Source
The starting material is a highly purified form of carbon, most commonly graphite. This is the same material found in a pencil lead. Graphite is chosen because it is an inexpensive and abundant source of carbon atoms.
The Diamond Seed
A tiny, pre-existing diamond crystal, often just a sliver, is placed in the growth cell. This seed crystal acts as a template or a blueprint. Without it, the carbon atoms would crystallize randomly; the seed ensures they arrange themselves in the correct diamond structure.
The Metal Catalyst
A mixture of metals, such as iron, nickel, or cobalt, is essential to the process. At high temperatures, these metals melt and act as a solvent for the carbon source. This molten metal bath is the medium through which the carbon atoms travel to reach the seed crystal.
The Specialized Press
The entire assembly is placed inside a massive mechanical press capable of generating extraordinary forces. These presses, such as a belt or cubic press, can create pressures exceeding 5.5 gigapascals (GPa)—equivalent to the pressure of a commercial jet balanced on the tip of your finger.
Simulating the Earth's Mantle: The Step-by-Step Method
The HPHT process is a carefully orchestrated sequence designed to manage immense forces and guide atomic-level construction.
Step 1: Precise Assembly
The diamond seed is placed at the bottom of a small capsule. The carbon source (graphite) is placed on top, and the entire mixture is surrounded by the metal catalyst powder. This capsule is then placed at the center of the press.
Step 2: Applying Extreme Conditions
The press applies immense pressure to the capsule, while an internal heating system raises the temperature to around 1,500°C (2,732°F). This combination of pressure and heat recreates the environment found over 100 miles below the Earth's surface.
Step 3: Dissolution and Crystallization
At this temperature, the metal catalyst melts, dissolving the graphite. A precise temperature difference is maintained between the hotter carbon source and the slightly cooler diamond seed. This gradient drives the dissolved carbon atoms to migrate through the molten metal towards the seed, where they precipitate out and bond to the crystal lattice.
Step 4: Controlled Cooling and Retrieval
Over several days or weeks, the diamond slowly grows around the seed. Once the desired size is reached, the system is carefully cooled, and the pressure is released. The newly formed synthetic diamond is then removed from the solidified metal.
Understanding the Trade-offs
While powerful, the HPHT method is not without its challenges and limitations. Understanding these trade-offs is key to appreciating why other methods, like Chemical Vapor Deposition (CVD), also exist.
High Energy Consumption
Generating and maintaining such extreme pressures and temperatures is incredibly energy-intensive. This is a significant factor in the operational cost of HPHT diamond synthesis.
Potential for Impurities
The metal catalyst is essential to the process, but trace amounts can sometimes become trapped within the diamond's crystal structure as it grows. These metallic inclusions can affect the diamond's clarity, color, and magnetic properties.
Comparison to CVD
The other primary method, Chemical Vapor Deposition (CVD), takes a fundamentally different approach. Instead of pressure, CVD uses a vacuum chamber filled with carbon-rich gases. This "bottom-up" method can produce diamonds with very high purity and offers different advantages for specific applications, particularly in electronics.
How to Apply This to Your Goal
Your interest in diamond synthesis dictates which aspects of the process are most relevant.
- If your primary focus is mimicking nature: The HPHT method is the closest technological parallel to the geological process that forms natural diamonds deep within the Earth.
- If your primary focus is industrial durability: HPHT is exceptionally good at producing tough, blocky diamond crystals ideal for abrasive, cutting, and drilling applications.
- If your primary focus is gemstone creation: Both HPHT and CVD produce high-quality gems, but HPHT diamonds may require post-growth treatment to improve color, while their growth patterns differ from those created via CVD.
Ultimately, the HPHT process is a remarkable feat of materials science, giving humanity the power to replicate one of nature's most extreme creative acts in a controlled laboratory setting.
Summary Table:
| Component | Role in HPHT Process |
|---|---|
| Carbon Source (Graphite) | Provides the raw carbon atoms for diamond growth |
| Diamond Seed | Acts as a template for the diamond crystal structure |
| Metal Catalyst (Fe, Ni, Co) | Dissolves carbon and facilitates its transport to the seed |
| Specialized Press | Generates extreme pressure (>5.5 GPa) and heat (~1500°C) |
Ready to explore high-performance materials for your laboratory? The precise control of pressure and temperature is key to advanced material synthesis. At KINTEK, we specialize in providing the robust lab equipment and consumables needed for cutting-edge research and industrial applications. Whether you're working on diamond synthesis or other high-pressure processes, our expertise can help you achieve reliable and repeatable results. Contact our experts today to discuss how KINTEK can support your laboratory's specific needs with tailored solutions.
Visual Guide
Related Products
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Automatic High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- 24T 30T 60T Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Heated Hydraulic Press Machine with Heated Plates Split Manual Laboratory Hot Press
- Heated Hydraulic Press Machine with Integrated Manual Heated Plates for Lab Use
People Also Ask
- What are heated hydraulic presses used for? Molding Composites, Vulcanizing Rubber, and More
- How much force can a hydraulic press exert? Understanding its immense power and design limits.
- How does a vacuum furnace environment influence sintered Ruthenium powder? Achieve High Purity and Theoretical Density
- Does a hydraulic press have heat? How Heated Platens Unlock Advanced Molding and Curing
- Why do you need to follow the safety procedure in using hydraulic tools? Prevent Catastrophic Failure and Injury



















