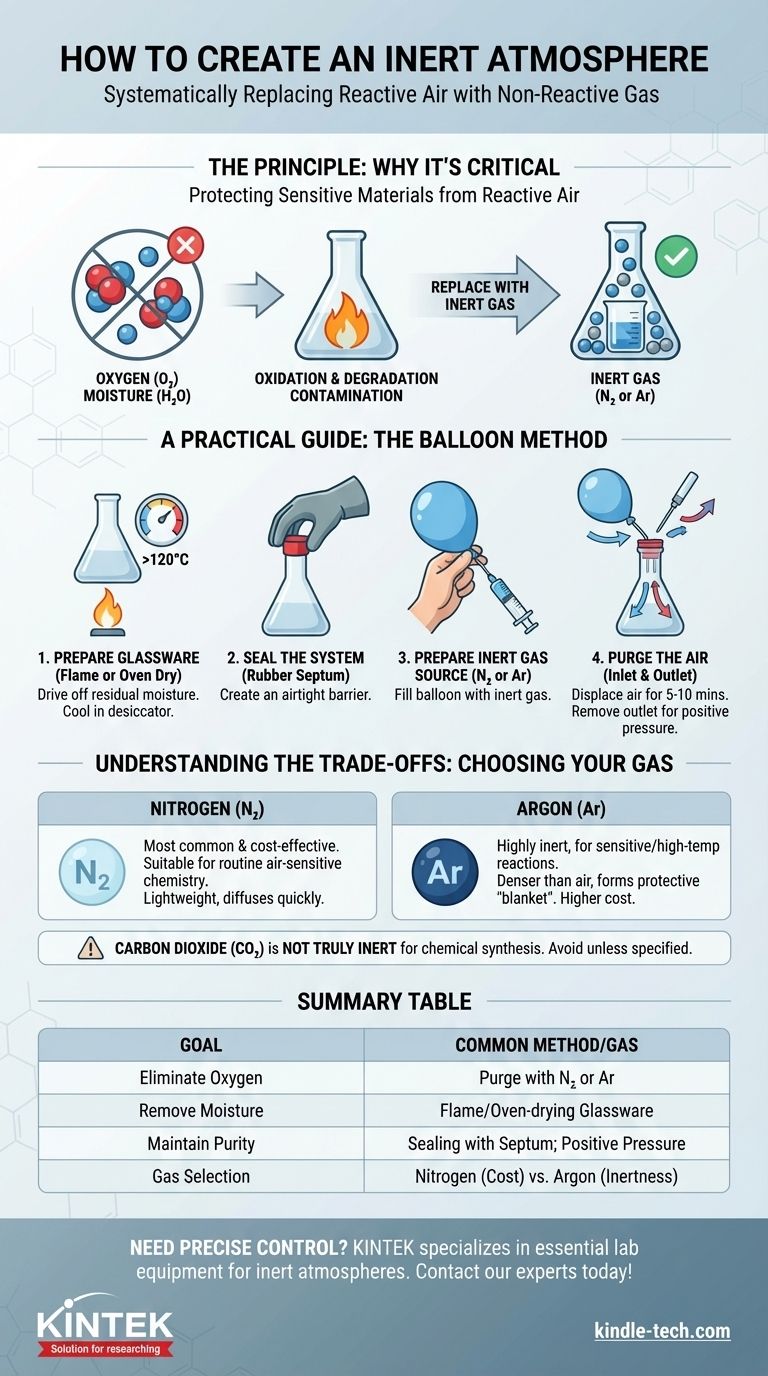
To create an inert atmosphere, you must systematically replace the reactive air inside a sealed container with a non-reactive gas. A common laboratory method involves flame-drying a reaction flask, sealing it with a rubber septum, and then using a balloon filled with nitrogen or argon to purge the air and maintain a positive pressure of the inert gas.
The fundamental goal is not simply to add an inert gas, but to actively remove reactive components like oxygen and moisture from your system. This protects sensitive materials and prevents unwanted side reactions, ensuring the stability and success of your process.

The Principle: Why an Inert Atmosphere is Critical
To properly implement an inert atmosphere, you must first understand what you are protecting your materials from. The air around us is a mixture of gases that are often highly reactive.
Eliminating Reactive Oxygen
The primary adversary is oxygen, which makes up about 21% of the air. It is a powerful oxidizing agent that readily participates in chemical reactions.
These oxidation processes can degrade sensitive reagents, create unwanted byproducts, or completely inhibit a desired chemical transformation. An inert gas physically displaces the oxygen, removing it from the equation.
Removing Residual Moisture
Water is another reactive compound present as moisture in the air and adsorbed onto the surfaces of glassware.
Flame-drying or oven-drying glassware before use is a critical step. This high heat drives off the microscopic layer of water, ensuring your system is not only oxygen-free but also dry.
Preventing Contamination and Hazard
Beyond specific chemical reactions, an inert atmosphere protects against general contamination. It also significantly reduces the risk of fire or explosions when working with flammable solvents or pyrophoric materials, which can ignite spontaneously on contact with air.
A Practical Guide: The Balloon Method
This technique is a simple, cost-effective way to establish an inert atmosphere for many common laboratory applications.
Step 1: Prepare Your Glassware
Begin by ensuring your reaction flask is scrupulously clean and dry. For sensitive reactions, you must flame-dry the flask under a vacuum or oven-dry it for several hours (typically at >120°C) and allow it to cool in a moisture-free environment like a desiccator.
Place a magnetic stir bar inside before you begin this process.
Step 2: Seal the System
Once the flask is cool to the touch (handle with thick gloves if necessary), immediately fold a rubber septum over the ground-glass joint. This creates an airtight seal that can be pierced by a needle.
Step 3: Prepare the Inert Gas Source
Fill a standard party balloon with your chosen inert gas, typically nitrogen or argon, to a diameter of about 7-8 inches. Twist the neck to prevent gas from escaping and attach a syringe needle.
Step 4: Purge the Air
To displace the air inside your flask, you need both an inlet and an outlet.
Insert the needle from your gas-filled balloon through the septum. Then, insert a second, "outlet" needle through the septum that is open to the atmosphere. This allows the heavier air to be pushed out as the lighter inert gas flows in. After 5-10 minutes of purging, you can remove the outlet needle. The balloon will maintain a slight positive pressure, ensuring that any potential leaks flow outward, preventing air from seeping in.
Understanding the Trade-offs: Choosing Your Gas
The gas you choose depends on your budget, the nature of your work, and the level of sensitivity required.
Nitrogen (N₂)
Nitrogen is the most common and cost-effective choice. It is suitable for the vast majority of routine air-sensitive chemistry. It is lightweight and diffuses quickly, making it effective for purging systems.
Argon (Ar)
Argon is significantly more inert than nitrogen and is used for highly sensitive reactions, particularly those involving organometallic reagents or high temperatures.
It is also about 1.5 times denser than air. This means it can form a protective "blanket" over a reaction mixture, making it excellent for techniques where a container is left open to the inert atmosphere. This performance comes at a higher cost.
Carbon Dioxide (CO₂)
While sometimes used for fire suppression or food preservation, CO₂ is not a truly inert gas for chemical synthesis. It can react with many reagents (especially strong bases) and should be avoided unless a specific protocol calls for it.
Making the Right Choice for Your Goal
Your application dictates the best approach and gas selection.
- If your primary focus is routine air-sensitive chemistry: Nitrogen is the standard, cost-effective choice for protecting most reactions.
- If your primary focus is highly sensitive materials or high-temperature processes: Argon provides a superior, denser, and more inert environment worth the extra cost.
- If your primary focus is fire suppression or food packaging: Carbon dioxide is a viable option, but it is not suitable for reactive chemical synthesis.
Ultimately, mastering inert atmosphere techniques gives you precise control over the chemical environment.
Summary Table:
| Key Component | Purpose | Common Method/Gas |
|---|---|---|
| Eliminate Oxygen | Prevent oxidation & unwanted reactions | Purging with N₂ or Ar |
| Remove Moisture | Protect hygroscopic materials | Flame-drying or oven-drying glassware |
| Maintain Purity | Ensure a stable, non-reactive environment | Sealing with a septum; positive pressure |
| Gas Selection | Balance cost and performance | Nitrogen (cost-effective) vs. Argon (highly inert) |
Need precise control over your chemical environment? KINTEK specializes in high-quality lab equipment and consumables essential for creating and maintaining perfect inert atmospheres. From durable reaction flasks and reliable septa to gas regulators, we provide the tools you need for successful air-sensitive work. Contact our experts today to discuss your specific laboratory requirements and ensure the stability of your most sensitive processes!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the standards for annealing? Mastering the Custom Thermal Recipe for Your Material
- Why is hydrogen used in furnaces? Achieve Superior Purity and Bright Finishes
- Why hydrogen gas is used in annealing process? Achieve a Bright, Oxide-Free Metal Finish
- What are atmosphere furnaces? Mastering Controlled Heat Treatment for Superior Materials
- What are the atmospheres for heat treatment? Master Surface Protection & Modification
- What is hydrogen annealing? Achieve Superior Material Properties with Bright Annealing
- What are the factors affecting the heat treatment process? Master Temperature, Time, Cooling & Atmosphere
- Why is an atmosphere-controlled sintering furnace necessary for ordered intermetallic nanocrystals? Essential Guide



















