In short, deposition is a physical process. It describes the direct transition of a substance from a gaseous state to a solid state, bypassing the liquid phase entirely. This change affects the physical form of the substance, not its underlying chemical composition.
Deposition is classified as a physical change because it only alters the arrangement and energy of molecules, not their internal structure or identity. The chemical bonds within the molecules remain intact throughout the process.
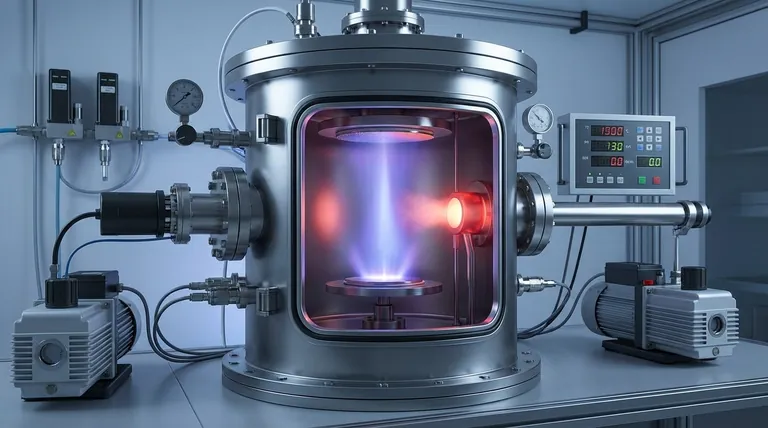
The Core Distinction: Physical vs. Chemical Change
To understand why deposition is a physical process, we must first establish the fundamental difference between physical and chemical changes. This distinction is central to chemistry and materials science.
What Defines a Physical Change?
A physical change alters the form or appearance of a substance but does not create a new substance. The molecules themselves are unchanged.
These changes primarily involve overcoming or creating intermolecular forces—the forces between molecules. Common examples include changes of state (melting, freezing, boiling), changes in shape, or mixing substances without a reaction.
What Defines a Chemical Change?
A chemical change, or chemical reaction, results in the formation of one or more entirely new substances with different chemical properties.
This process involves the breaking of existing chemical bonds and the formation of new ones. The atoms are rearranged to create new molecules. Indicators often include a color change, the production of a gas, or the formation of a precipitate from a solution.
Applying the Framework to Deposition
With this framework, we can clearly analyze the process of deposition.
Deposition: A Change in Arrangement, Not Identity
Deposition is a phase transition. Consider the example of water vapor in cold air turning directly into frost on a window.
The water molecules (H₂O) in the gaseous state are far apart and move randomly. During deposition, they lose energy, slow down, and arrange themselves into a highly ordered, crystalline structure (ice). The substance is still H₂O—only its physical state has changed.
The Role of Energy
Deposition is an exothermic process, meaning it releases energy into the surroundings. The high-energy gas particles must release thermal energy to settle into the low-energy, stable arrangement of a solid.
This energy change affects the kinetic energy of the molecules, not the bond energy within the molecules.
An Important Contrast: Chemical Vapor Deposition (CVD)
A common point of confusion is the process of Chemical Vapor Deposition (CVD), which is widely used in the semiconductor industry.
While CVD results in a solid film being deposited from a gas, it is a chemical process. In CVD, precursor gases react on a surface, and the product of that chemical reaction is what forms the solid layer. A new material is created, unlike in physical deposition.
Common Misconceptions
Understanding the nuances helps avoid common classification errors.
Why It Can Seem Chemical
The formation of a solid can sometimes appear to be a chemical reaction, similar to precipitation. However, the key difference is the identity of the solid. In deposition, the solid is the same substance as the gas. In precipitation, the solid is a new compound formed from a reaction between dissolved ions.
The Opposite Process: Sublimation
Deposition has a direct opposite: sublimation, the transition from a solid directly to a gas. Like deposition, sublimation is also a physical change. A block of dry ice (solid CO₂) turning into CO₂ gas is a perfect example.
How to Identify the Type of Change
Use this simple guide to determine whether a process is physical or chemical.
- If your primary observation is a change of state (solid to liquid, gas to solid, etc.): This is a physical change, as long as the substance's chemical formula remains the same before and after.
- If your primary observation is the creation of a new substance (indicated by a permanent color change, fizzing, or rust): This is a chemical change involving the formation of new chemical bonds.
- If the process involves both (like Chemical Vapor Deposition): The process is fundamentally chemical, as a reaction is required to produce the new material that is then physically deposited.
Ultimately, the distinction always comes down to one question: has the fundamental chemical identity of the substance been altered?
Summary Table:
| Aspect | Physical Change (Deposition) | Chemical Change (e.g., CVD) |
|---|---|---|
| Molecular Identity | Unchanged | Altered (new substance formed) |
| Bonds Affected | Intermolecular forces | Chemical bonds (broken/formed) |
| Energy Change | Exothermic (releases heat) | Can be endothermic or exothermic |
| Example | Water vapor → Frost | Precursor gases → Silicon film |
Need precise control over physical and chemical processes in your lab? KINTEK specializes in high-quality lab equipment and consumables, including systems for deposition and material synthesis. Whether you're conducting fundamental research on phase transitions or developing advanced materials with Chemical Vapor Deposition, our solutions ensure accuracy and reliability. Contact our experts today to find the perfect equipment for your laboratory's unique needs!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the industrial application of graphite as lubricant? A Guide to Extreme Heat & Pressure Solutions
- What is sputtering technique for thin films? A Guide to Precision Coating Technology
- What are the defects of sintered metal? Understanding Porosity, Cracking & Distortion
- What is the strength of the magnetron magnetic field? It's About Function, Not Just Force
- What role does a laboratory high-precision oven play in bioreactor stability? Ensure High-Pressure Accuracy
- What is the development of thin film? From 17th Century Experiments to Atomic-Scale Engineering
- What is meant by uniformity of the film? The Key to Reliable Thin-Film Performance
- What are the different types of frames in compression? A Guide to I, P, and B-Frames



















