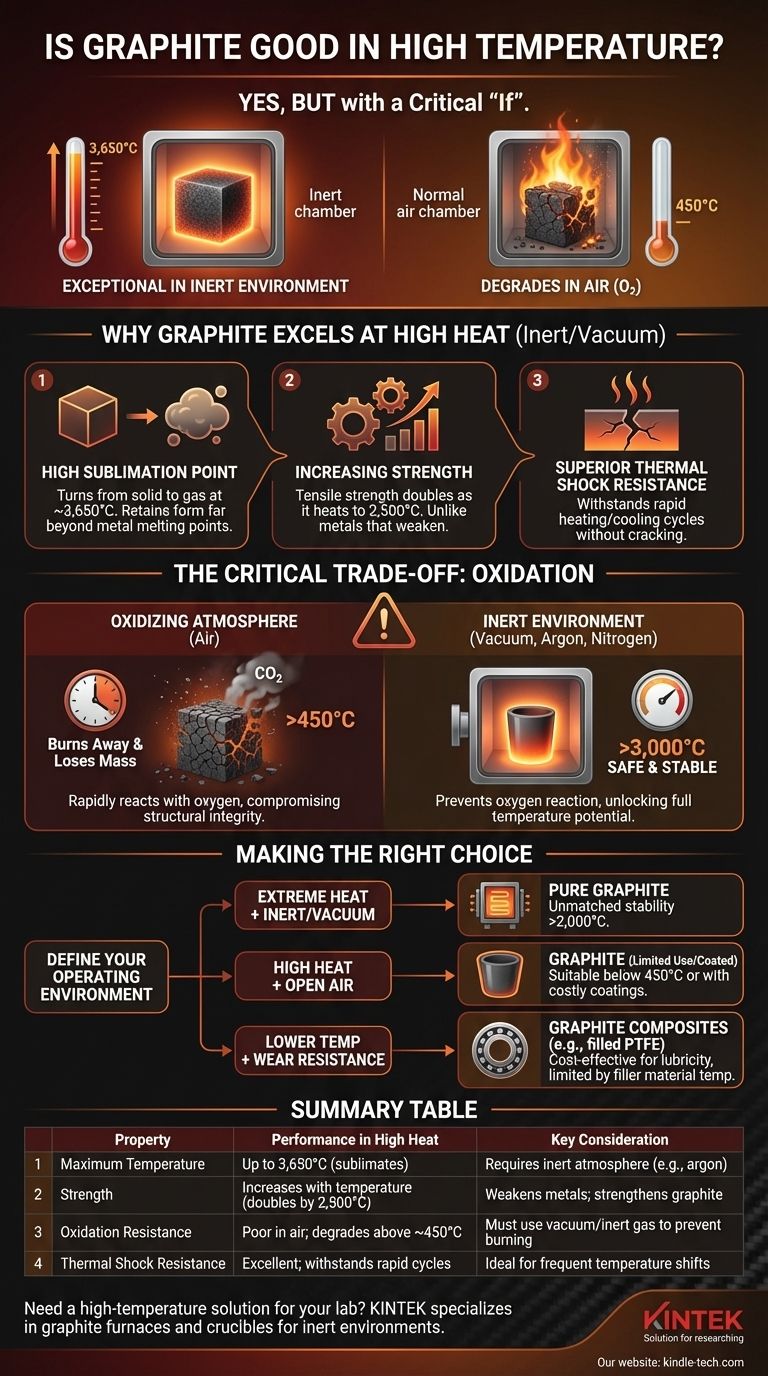
In short, yes. Graphite is an exceptional material for high-temperature applications due to its incredibly high sublimation point and its unique ability to gain strength as it gets hotter. However, its performance is critically dependent on the surrounding atmosphere, as it will rapidly degrade in the presence of oxygen at much lower temperatures.
Graphite's suitability for high heat presents a paradox. While it remains structurally sound at temperatures that would melt most metals, its practical use is often limited not by its melting point, but by its reaction with oxygen in the air.

Why Graphite Excels at High Temperatures
Graphite's atomic structure gives it a set of thermal properties that are superior to almost any common metal or ceramic in specific conditions.
Exceptionally High Sublimation Point
Unlike most materials that melt into a liquid, graphite sublimates, turning directly from a solid to a gas. This transition occurs at an extremely high temperature, around 3,650°C (6,602°F).
This means it retains its solid form and structural integrity at temperatures far beyond the melting point of steel, aluminum, or even tungsten.
Increasing Strength with Heat
One of graphite's most remarkable and counter-intuitive properties is that its tensile strength increases with temperature. It roughly doubles its room-temperature strength as it heats up to 2,500°C (4,532°F).
Metals, in contrast, become progressively weaker and softer as they are heated. This makes graphite uniquely suited for high-temperature structural components like furnace elements and crucibles.
Superior Thermal Shock Resistance
Graphite can withstand rapid changes in temperature without cracking or failing. This property, known as thermal shock resistance, is a result of its high thermal conductivity and low coefficient of thermal expansion.
Its ability to allow for rapid heating and cooling cycles, as noted in manufacturing processes, significantly reduces production time and the risk of material failure.
The Critical Trade-off: Oxidation
The single greatest limitation of using graphite at high temperatures is its reaction with oxygen. This factor is non-negotiable and must be the primary consideration in any design.
The Role of an Oxidizing Atmosphere
In a standard air environment, graphite begins to oxidize and lose mass at temperatures starting around 450°C (842°F). The rate of this degradation accelerates rapidly as the temperature increases.
Effectively, the graphite "burns" away, converting to carbon dioxide (CO₂) gas. This reaction compromises its structural integrity and leads to component failure.
The Importance of an Inert Environment
To leverage graphite's full temperature potential, it must be used in a vacuum or an inert atmosphere. Environments filled with gases like argon or nitrogen prevent oxygen from reacting with the carbon.
In these controlled environments, graphite components can be safely and reliably used right up to their sublimation temperature of over 3,000°C.
Understanding Different Material Forms
It is also crucial to distinguish pure graphite from graphite composites. For example, graphite-filled PTFE is a material where graphite powder is added to a plastic (PTFE) to improve wear resistance.
While this composite has excellent sliding characteristics, its temperature limit is dictated by the PTFE, which degrades at much lower temperatures than pure graphite.
Making the Right Choice for Your Application
To determine if graphite is the correct material, you must first define your operating environment.
- If your primary focus is extreme heat in a vacuum or inert gas: Graphite is one of the best materials available, offering unmatched structural stability well above 2,000°C.
- If your primary focus is high heat in open air: Graphite is only suitable for moderate temperatures (below 450°C) unless you can implement specialized anti-oxidation coatings, which add complexity and cost.
- If your primary focus is wear resistance and lubricity at lower temperatures: A graphite-filled composite may be a more suitable and cost-effective choice than a pure graphite component.
Ultimately, successfully using graphite depends on aligning its unique properties with the precise demands of its intended environment.
Summary Table:
| Property | Performance in High Heat | Key Consideration |
|---|---|---|
| Maximum Temperature | Up to 3,650°C (sublimates) | Requires an inert atmosphere (e.g., argon) |
| Strength | Increases with temperature (doubles by 2,500°C) | Weakens metals; strengthens graphite |
| Oxidation Resistance | Poor in air; degrades above ~450°C | Must be used in a vacuum or inert gas to prevent burning |
| Thermal Shock Resistance | Excellent; withstands rapid heating/cooling | Ideal for applications with frequent temperature cycles |
Need a high-temperature solution for your lab?
Graphite's performance is unmatched in controlled environments. KINTEK specializes in high-temperature lab equipment, including graphite furnaces and crucibles designed for inert atmospheres. Our experts can help you select the right materials to ensure safety, efficiency, and reliability in your most demanding applications.
Contact our team today to discuss how our graphite-based solutions can meet your extreme heat challenges.
Visual Guide
Related Products
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How does a heat-collecting constant temperature heating magnetic stirrer contribute to wood delignification?
- Why is temperature excursion alarming important in ultra-low freezers? Protect Your Valuable Samples from Catastrophic Loss
- What is the pyrolysis method of disposal? Transforming Waste into Valuable Resources
- How does a sputtering system work? Achieve Superior Thin-Film Deposition for Your Lab
- Can FTIR be used for quantitative analysis? Yes, Here's How to Measure Concentration Accurately
- Why is ultrasonic dispersion equipment utilized for coal fly ash zeolite? Achieve Superior Nanoscale Homogenization
- What is the demand for synthetic diamonds? Rising Popularity for Ethical & Affordable Gems
- What is the hottest type of furnace? Discover the Unmatched Power of Electric Arc Furnaces



















