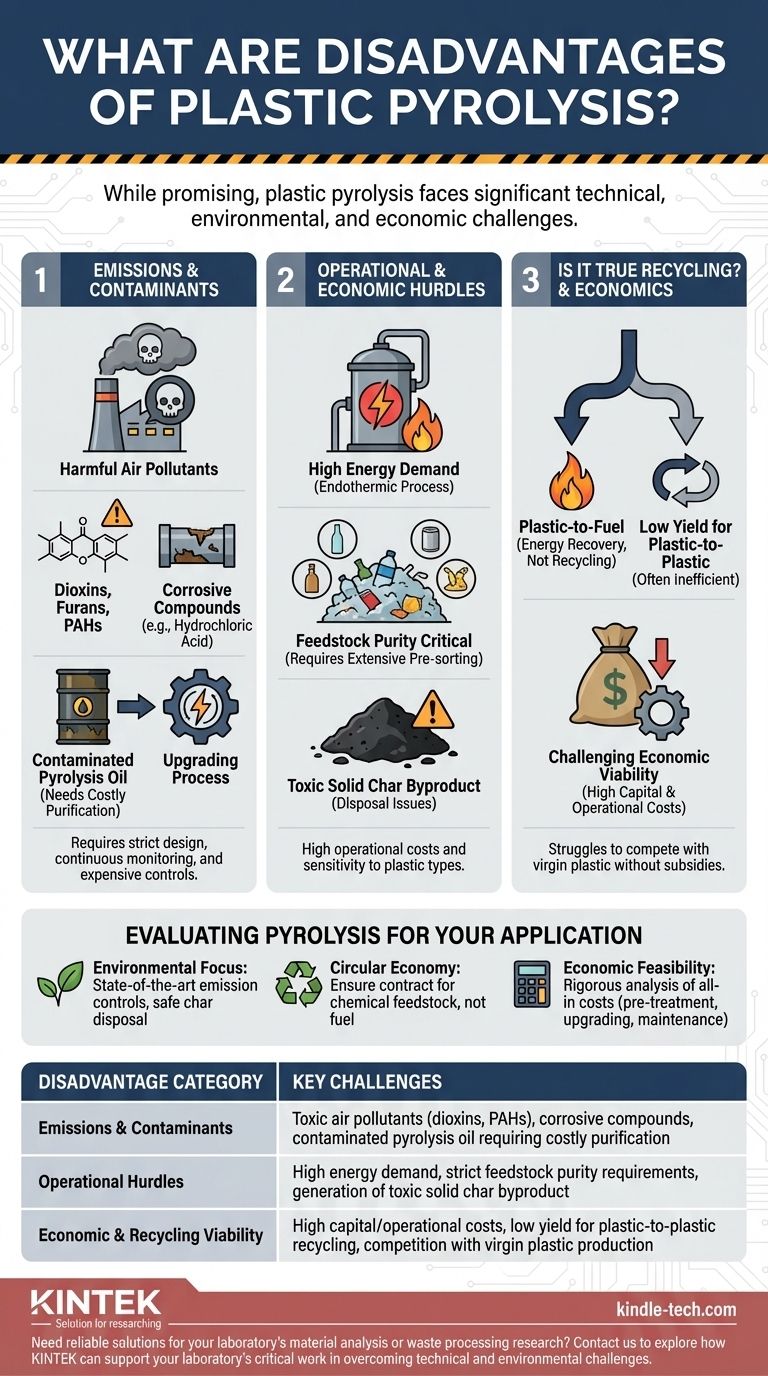
While a promising technology, plastic pyrolysis presents significant disadvantages that challenge its viability as a truly sustainable solution. The primary drawbacks include high energy requirements, the potential for generating toxic emissions and byproducts, and a strong sensitivity to the purity of the plastic feedstock. Without sophisticated and costly control systems, the process can create environmental problems rather than solve them.
Pyrolysis is not a simple "magic bullet" for plastic waste. Its environmental and economic success is entirely dependent on managing its inherent complexities, from controlling air pollution to purifying the final oil product, which adds substantial cost and technical hurdles.

The Challenge of Emissions and Contaminants
The core of pyrolysis involves breaking down plastics with heat in an oxygen-starved environment. This process, if not perfectly controlled, can release or create a host of undesirable substances.
Potential for Harmful Air Pollutants
Heating mixed plastics, especially those containing chlorine like PVC, can form highly toxic compounds. These include dioxins, furans, and polycyclic aromatic hydrocarbons (PAHs), which are potent air pollutants and carcinogens.
Proper design and continuous monitoring of the reactor and its exhaust gas treatment systems are essential to capture these emissions, as noted in process safety analyses. Failure to do so negates the environmental benefits.
Formation of Corrosive Compounds
Contaminants in the plastic waste stream, particularly chlorine from PVC or bromine from flame retardants, create significant operational problems. At high temperatures, these elements can form acids like hydrochloric acid.
This acid is extremely corrosive, damaging reactors, pipes, and other equipment. This necessitates the use of expensive, corrosion-resistant alloys, increasing the capital cost of the facility and leading to higher maintenance burdens.
Contaminated Pyrolysis Oil
The liquid product, known as pyrolysis oil or "py-oil," is not a direct substitute for crude oil. It is often contaminated with chlorine, sulfur, oxygen, and heavy metals that were present in the original plastic waste.
This oil must undergo significant, energy-intensive secondary processing—such as hydrotreating—to remove these impurities before it can be used in a conventional refinery. This "upgrading" step adds another layer of cost and complexity.
Operational and Economic Hurdles
Beyond the chemical challenges, the day-to-day operation and economic model of pyrolysis plants face major obstacles.
High Energy Demand
Pyrolysis is an endothermic process, meaning it requires a constant and significant input of energy to maintain the high temperatures (typically 300-900°C) needed to break down plastic polymers.
While some of the non-condensable gases produced can be burned to help power the reactor, the overall process often has a high energy footprint. This must be factored into any life-cycle assessment of its net environmental impact.
Feedstock Purity is Critical
Pyrolysis systems perform best with a clean, homogeneous stream of specific plastics (like polyethylene and polypropylene). However, real-world municipal plastic waste is a messy, mixed jumble.
Contaminants like food residue, paper, glass, metal, and other plastic types (especially PVC) can disrupt the chemical reactions, reduce oil yield, and create the harmful byproducts mentioned earlier. This means extensive and costly pre-sorting, washing, and shredding are required.
Generation of Unwanted Byproducts
The process does not convert 100% of the plastic into usable oil. It also creates a solid carbonaceous residue, or char, and non-condensable gases.
The char can contain heavy metals and other toxic substances, concentrating them into a solid waste that may require disposal in a specialized landfill. While sometimes marketed as a product ("agri-char"), its contamination often makes this unfeasible.
Understanding the Trade-offs: Is It True Recycling?
A major point of contention is whether pyrolysis should be classified as "recycling" at all. The distinction has significant policy and environmental implications.
The "Plastic-to-Fuel" Pathway
In many existing and proposed plants, the primary use for the py-oil is to be burned as fuel. Critics argue this is not recycling but a form of energy recovery.
According to the established waste hierarchy, true recycling (which creates new materials) is environmentally preferable to energy recovery (which destroys the material for its energy content).
Low Yield for "Plastic-to-Plastic"
Achieving a true circular, "plastic-to-plastic" loop is the ultimate goal. However, the actual yield of high-quality chemical feedstock suitable for making new plastics is often low due to process inefficiencies and contamination.
A significant portion of the initial material is often lost as low-value byproducts, process energy, or heavily contaminated oils that can only be used as a low-grade fuel.
Challenging Economic Viability
The combination of high capital costs (reactor, emission controls), high operational costs (energy, maintenance, feedstock preparation), and the need for secondary processing of the oil makes the economics of pyrolysis difficult. Without significant government subsidies or high landfill taxes, many facilities struggle to compete with conventional plastic production from virgin fossil fuels.
Evaluating Pyrolysis for Your Application
To make an informed decision, you must weigh the technology's potential against its demonstrable disadvantages.
- If your primary focus is environmental impact: Prioritize facilities with state-of-the-art emission controls, transparent reporting on air quality, and a clear, safe disposal plan for contaminated char.
- If your primary focus is creating a circular economy: Scrutinize the facility's actual "plastic-to-plastic" yield and ensure the output is contractually destined for use as a chemical feedstock, not just burned for fuel.
- If your primary focus is economic feasibility: Demand a rigorous analysis of the all-in costs, including feedstock pre-treatment, secondary oil upgrading, and long-term maintenance of the reactor.
Plastic pyrolysis holds potential, but its success as a sustainable solution depends entirely on overcoming these formidable technical, environmental, and economic challenges.
Summary Table:
| Disadvantage Category | Key Challenges |
|---|---|
| Emissions & Contaminants | Toxic air pollutants (dioxins, PAHs), corrosive compounds, contaminated pyrolysis oil requiring costly purification |
| Operational Hurdles | High energy demand, strict feedstock purity requirements, generation of toxic solid char byproduct |
| Economic & Recycling Viability | High capital/operational costs, low yield for plastic-to-plastic recycling, competition with virgin plastic production |
Need reliable solutions for your laboratory's material analysis or waste processing research? At KINTEK, we specialize in providing high-performance lab equipment and consumables tailored to complex processes like pyrolysis. Whether you're analyzing emissions, testing materials, or developing sustainable technologies, our tools deliver the precision and durability you need. Contact us today to explore how KINTEK can support your laboratory's critical work in overcoming technical and environmental challenges.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Boron Nitride (BN) Ceramic Rod for High Temperature Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Wall Mounted Water Distillation Unit
People Also Ask
- Is pyrolysis process environmentally friendly? Unlocking Waste-to-Value with Responsible Technology
- What is the optimum temperature for pyrolysis? Target Your Desired Biochar, Bio-oil, or Syngas
- What are the components of a rotary kiln? A Guide to the Core Systems and Parts
- What is a rotary retort furnace? Achieve Superior Uniformity in Continuous Heat Treatment
- How do you make biochar pyrolysis? A Guide to Converting Biomass into Stable Carbon
- What is the capacity of a rotary furnace? Choose Between Batch or Continuous Processing
- What is the structure of a rotary hearth furnace? A Guide to Continuous, Uniform Heating
- What are the disadvantages of spray pyrolysis? Key Challenges for Thin-Film Quality

















