Ultimately, while X-ray Fluorescence (XRF) is a powerful, fast, and often non-destructive analytical technique, its primary drawbacks stem from fundamental physical limitations and its extreme sensitivity to the sample's condition. It struggles to detect very light elements, cannot provide information about a chemical's form, and its accuracy is highly dependent on proper sample preparation and calibration.
XRF is a superb tool for identifying what elements are present and in what quantity for most of the periodic table, but it cannot tell you how those elements are chemically bonded and can be easily misled by a poorly prepared sample or complex matrix.

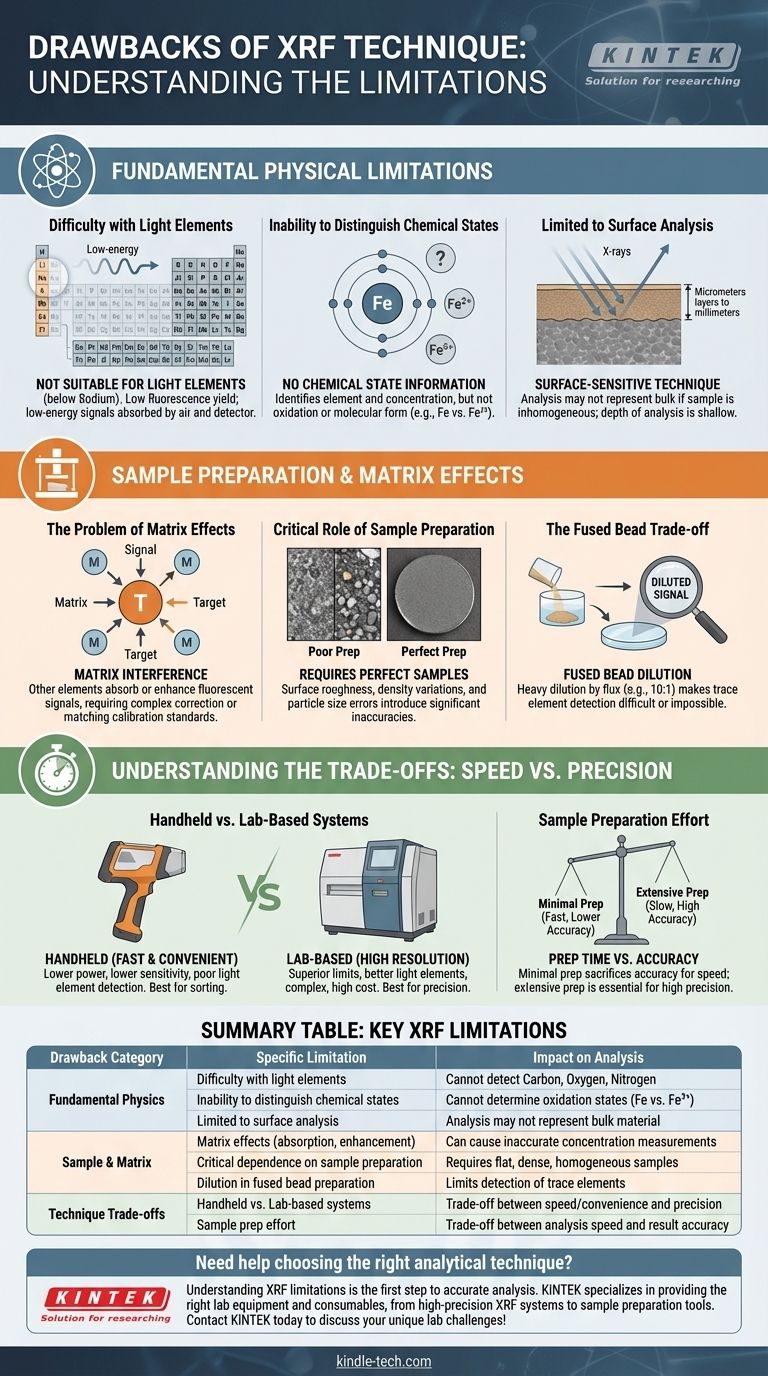
Fundamental Physical Limitations
The physics behind how X-rays interact with matter imposes several inherent constraints on the XRF technique. These are not issues you can solve with better instrumentation alone.
Difficulty with Light Elements
XRF is generally not suitable for elements lighter than sodium (Na, atomic number 11).
This is due to two reasons. First, lighter elements have a very low fluorescence yield, meaning they are inefficient at producing measurable X-ray signals. Second, the characteristic X-rays they do emit are very low-energy and are easily absorbed by the air, the detector window, and even the sample itself before they can be measured.
Inability to Distinguish Chemical States
XRF identifies the presence and concentration of an element, but it provides no information about its oxidation state or molecular form.
For example, XRF can tell you the total concentration of iron in a sample, but it cannot distinguish between metallic iron (Fe), ferrous iron (Fe²⁺), or ferric iron (Fe³⁺). For this, you would need a different technique like X-ray Photoelectron Spectroscopy (XPS) or Mössbauer spectroscopy.
Limited to Surface Analysis
XRF is a surface-sensitive technique, not a true bulk analysis method.
The primary X-rays from the instrument only penetrate a limited depth into the sample, typically from a few micrometers to several millimeters, depending on the sample's density and the X-ray energy. The resulting fluorescent X-rays can only escape from this same shallow depth. This means the analysis may not be representative of the entire bulk material if the sample is not homogeneous.
Sample Preparation and Matrix Effects
Beyond its physical limits, the greatest source of error and frustration in XRF analysis comes from the sample itself. The technique's accuracy is critically dependent on how the sample is prepared and what other elements are present.
The Problem of Matrix Effects
The "matrix" refers to everything in the sample that is not the specific element being measured. These other elements can absorb or enhance the fluorescent signal from your element of interest, leading to inaccurate results.
For example, high concentrations of iron in a sample can absorb the fluorescent X-rays from nickel, making the measured nickel concentration appear lower than it actually is. These effects must be corrected with sophisticated software or by using calibration standards that closely match the sample's matrix.
The Critical Role of Sample Preparation
For quantitative analysis, the sample presented to the instrument must be perfectly flat, dense, and homogeneous.
Surface roughness, variations in particle size, and inconsistent density can all scatter the X-ray signal unpredictably and introduce significant errors. This is why samples are often ground into a fine powder and pressed into a pellet.
The Fused Bead Trade-off
To overcome matrix and particle size effects, a common method is to create a fused bead, where the sample is dissolved in a lithium borate flux at high temperature to form a homogeneous glass disk.
However, as the reference material correctly points out, this method has a significant drawback. The sample is heavily diluted by the flux, often at a 10:1 ratio. This process makes it impossible to detect elements present at very low (trace) concentrations, as their signal is diluted below the instrument's detection limit.
Understanding the Trade-offs: Speed vs. Precision
Choosing to use XRF, and how you use it, involves a series of trade-offs. Understanding them is key to getting reliable data.
Handheld vs. Lab-Based Systems
Portable (Handheld) XRF analyzers offer incredible speed and convenience for field use. However, they typically have lower power, less sensitive detectors, and cannot create the vacuum needed to measure light elements effectively. They are excellent for sorting and screening but less accurate for precise quantitative work.
Lab-based WDXRF (Wavelength Dispersive XRF) systems offer far superior resolution, lower detection limits, and better light element performance. The trade-off is their high cost, complexity, and the need for a controlled lab environment and skilled operators.
Sample Preparation Effort
Minimal sample prep (e.g., analyzing a rock or metal part "as is") is fast but risks significant inaccuracy due to surface effects and inhomogeneity.
Extensive sample prep (e.g., grinding, pressing a pellet, or creating a fused bead) takes much more time and effort but is essential for achieving the high accuracy and precision required for quality control or research.
Is XRF the Right Technique for Your Goal?
Before committing to XRF, consider your primary objective.
- If your primary focus is rapid material sorting and identification: A handheld XRF is an ideal tool, providing "good enough" data in seconds, despite its lower precision.
- If your primary focus is high-accuracy composition of major and minor elements (e.g., in cement, geology, or metals): A lab-based WDXRF or high-end EDXRF with rigorous sample preparation (like pressed pellets or fused beads) is the gold standard.
- If your primary focus is detecting trace elements (parts-per-million level): XRF is likely the wrong choice due to its detection limits; you should consider techniques like Inductively Coupled Plasma (ICP-MS or ICP-OES).
- If your primary focus is understanding chemical bonding or oxidation states: XRF cannot provide this information, and you must use a different technique like XPS, Raman, or XRD.
Choosing the right analytical tool is about aligning the technique's capabilities and limitations with your specific question.
Summary Table:
| Drawback Category | Specific Limitation | Impact on Analysis |
|---|---|---|
| Fundamental Physics | Difficulty with light elements (below Sodium) | Cannot detect elements like Carbon, Oxygen, Nitrogen |
| Inability to distinguish chemical states | Cannot determine oxidation states (e.g., Fe vs. Fe²⁺) | |
| Limited to surface analysis | Analysis may not represent bulk material if inhomogeneous | |
| Sample & Matrix | Matrix effects (absorption, enhancement) | Can cause inaccurate concentration measurements |
| Critical dependence on sample preparation | Requires flat, dense, homogeneous samples for accuracy | |
| Dilution in fused bead preparation | Limits detection of trace elements | |
| Technique Trade-offs | Handheld vs. Lab-based systems | Trade-off between speed/convenience and precision/accuracy |
| Sample prep effort | Trade-off between analysis speed and result accuracy |
Need help choosing the right analytical technique for your lab?
Understanding the limitations of XRF is the first step to accurate material analysis. KINTEK specializes in providing the right lab equipment and consumables to meet your specific needs, whether you require high-precision XRF systems, sample preparation tools for pressed pellets, or guidance on alternative techniques like XRD or XPS.
Our experts can help you select the ideal solution to ensure reliable and efficient results for your laboratory's unique challenges.
Contact KINTEK today to discuss your application and find the perfect analytical solution!
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- Why is an airtight sample holder with a beryllium window required for XRD of sulfide solid electrolytes?
- How should a sample holder be handled to ensure its longevity? Protect Your Lab Investment and Data Integrity
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference
- What are the factors that affect melting and boiling point? Unlock the Science of Phase Transitions
- What is the difference between XRF and XRD techniques? A Guide to Choosing the Right Analytical Tool







