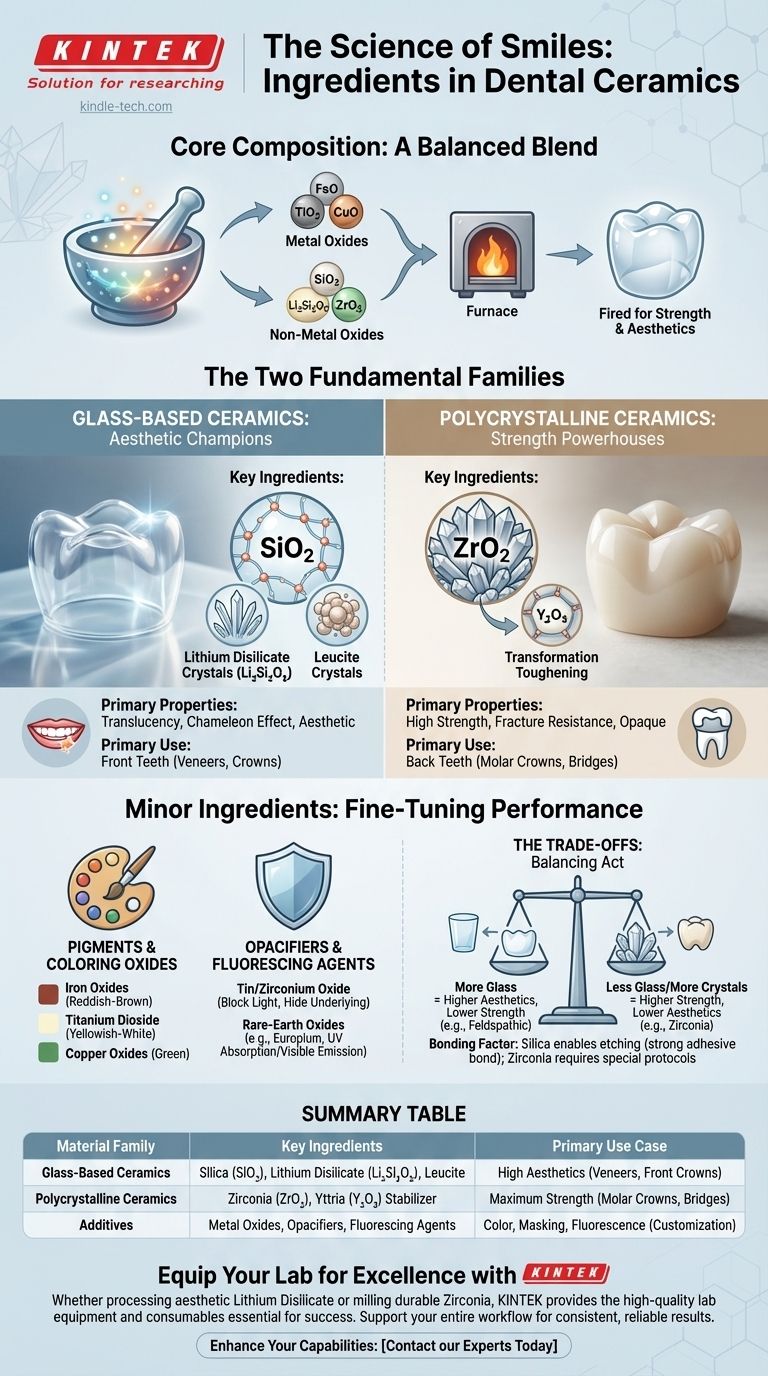
At their core, dental ceramics are primarily composed of inorganic, non-metallic materials. The specific "ingredients" are a carefully selected blend of metal and non-metal oxides that are fired at high temperatures to create a hard, stable, and biocompatible final product. The exact composition varies significantly, as "dental ceramic" is a broad category, not a single substance.
The essential takeaway is that dental ceramics are not one material, but a family of materials built on a spectrum. The choice of ingredients is a deliberate engineering decision to balance the trade-off between aesthetic beauty (driven by glass content) and mechanical strength (driven by crystalline content).

The Two Fundamental Families of Dental Ceramics
To understand the ingredients, you must first understand that modern dental ceramics are divided into two main classes based on their microstructure. This division dictates their primary ingredients, properties, and clinical use.
Glass-Based Ceramics: The Aesthetic Champions
These materials contain a significant amount of a glass matrix, which is what gives them their lifelike translucency and chameleon-like ability to blend in with natural teeth.
The foundational ingredient is a silica (silicon dioxide, SiO₂) glass network. Traditional feldspathic porcelains use feldspar, a naturally occurring mineral containing potassium and aluminum silicates.
To improve strength, reinforcing crystalline fillers are added. The type of filler defines the specific material:
- Leucite-reinforced ceramics add leucite crystals to the glass, improving resistance to fracture.
- Lithium disilicate ceramics (e.g., IPS e.max) are the most popular in this class. They contain a high concentration of needle-like lithium disilicate (Li₂Si₂O₅) crystals, which dramatically increases strength while maintaining excellent translucency.
Polycrystalline Ceramics: The Strength Powerhouses
These materials have no glass matrix at all. Instead, they are composed of very densely packed crystals, making them exceptionally strong and fracture-resistant, but also inherently more opaque.
The primary ingredient is a high-strength metal oxide.
- Zirconia (zirconium dioxide, ZrO₂) is the dominant material in this category. It is the strongest ceramic available for dentistry.
- To prevent cracking under stress, a stabilizing ingredient like yttria (yttrium oxide, Y₂O₃) is added. This stabilizer enables a phenomenon called "transformation toughening," where the crystal structure actually changes at the tip of a propagating crack to stop it in its tracks.
The Minor Ingredients: Fine-Tuning Performance and Appearance
Beyond the core structural components, a variety of oxides are added in small quantities to control the final look and behavior of the restoration.
Pigments and Coloring Oxides
These are metal oxides added to the ceramic powder or applied as surface stains to mimic the subtle shades of natural teeth.
- Iron oxides create reddish-brown hues.
- Titanium dioxide creates yellowish-white shades.
- Copper oxides can produce green tones.
Opacifiers and Fluorescing Agents
These ingredients manage how the ceramic interacts with light.
- Opacifiers like tin oxide or zirconium oxide are added to block light, which is necessary to hide a dark underlying tooth or a metal post.
- Fluorescing agents, such as rare-earth oxides like europium, are added to make the ceramic absorb UV light and emit it as visible light, perfectly mimicking the natural fluorescence of enamel.
Understanding the Trade-offs: Strength vs. Aesthetics
The choice of ingredients is a calculated compromise. No single ceramic is perfect for every situation.
The Glass-Matrix Dilemma
The very ingredient that makes glass-based ceramics beautiful—the glass—is also their primary weakness. The more glass content, the higher the translucency, but the lower the mechanical strength. This is why feldspathic porcelain is beautiful but brittle, while lithium disilicate is a good compromise.
The Zirconia Conundrum
The incredible strength of zirconia comes from its dense, glass-free crystal structure. This makes it naturally opaque. Newer "high translucency" zirconias have been developed for better aesthetics, but this is achieved by altering the crystal size and yttria content, which slightly reduces their maximum flexural strength compared to the most opaque, high-strength versions.
The Bonding Factor
The ingredients directly impact how a crown is secured in the mouth. The silica in glass-based ceramics can be etched with hydrofluoric acid, creating a microscopic honeycomb surface that allows for a very strong adhesive bond to the tooth. Zirconia lacks silica and cannot be etched in the same way, requiring different bonding protocols and specialized primers to achieve a reliable long-term bond.
Making the Right Choice for Your Goal
Understanding these material families allows dentists to select the ideal material based on the specific clinical demands of the patient.
- If your primary focus is maximum aesthetics (e.g., a front tooth veneer): A glass-based ceramic like lithium disilicate is often the ideal choice for its superior translucency and lifelike appearance.
- If your primary focus is maximum strength (e.g., a molar crown or a multi-tooth bridge): A polycrystalline ceramic like yttria-stabilized zirconia is the definitive standard for its exceptional durability and fracture resistance.
- If your primary focus is biocompatibility: All-ceramic restorations, regardless of the family, are highly biocompatible and are the best solution for patients with known metal allergies or sensitivities.
Ultimately, the ingredients in dental ceramics are precisely chosen to provide a customized solution, ensuring the right balance of strength and beauty for every part of your smile.
Summary Table:
| Material Family | Key Ingredients | Primary Use Case |
|---|---|---|
| Glass-Based Ceramics | Silica (SiO₂) Glass, Leucite, Lithium Disilicate (Li₂Si₂O₅) | High aesthetics for front teeth (veneers, crowns) |
| Polycrystalline Ceramics | Zirconia (ZrO₂), Yttria (Y₂O₃) stabilizer | Maximum strength for back teeth (molar crowns, bridges) |
| Additives | Metal Oxides (for color), Opacifiers (e.g., Tin Oxide) | Customizing shade and masking underlying structures |
Ready to find the perfect ceramic material for your laboratory's next case?
KINTEK specializes in providing high-quality lab equipment and consumables for dental laboratories. Whether you are processing strong, aesthetic lithium disilicate or milling durable zirconia, having the right tools is critical for success. Our products support the entire workflow, ensuring consistent, reliable results for your dental restorations.
Let us help you enhance your lab's capabilities. Contact our experts today to discuss your specific needs!
Visual Guide
Related Products
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
- Boron Nitride (BN) Ceramic Tube
- Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
- High Purity Zinc Foil for Battery Lab Applications
People Also Ask
- What is the type of silicon carbide? A Guide to Polymorphs, Grades, and Applications
- What is the temperature for ceramic parts sintering? A Guide to Material-Specific Sintering Cycles
- What physical changes occur during sintering of ceramic powders? Master the Transformation to Dense, Strong Parts
- What is microwave sintering? Unlock Faster, More Efficient Material Processing
- What are ceramic and its applications? From Pottery to Spacecraft, Unlocking Material Potential
- Why ceramics can withstand high temperature? Unlock the Secrets of Atomic Structure
- Why are ceramics used for furnace lining? Achieve Peak Efficiency and Durability
- What type of structure is silicon carbide? A Covalent Network Solid for Extreme Performance

















