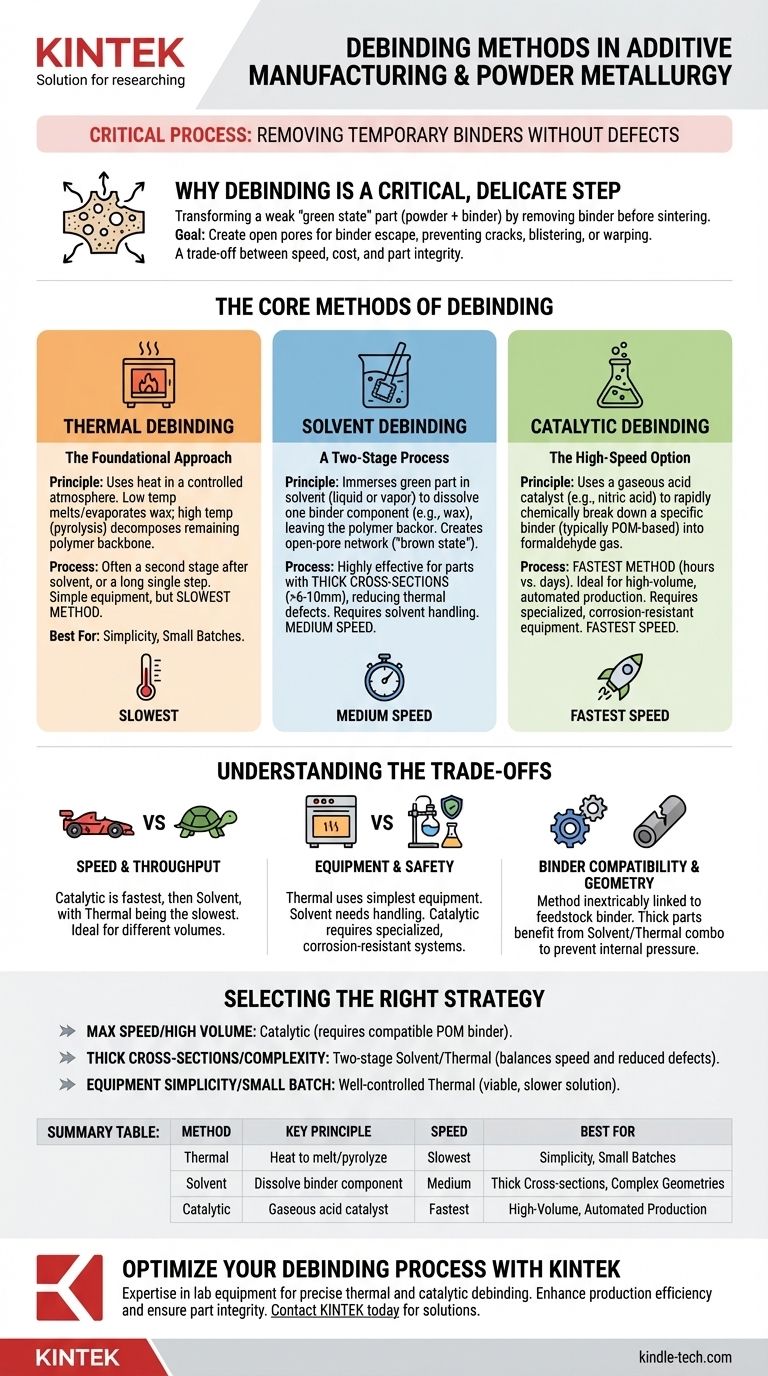
In additive manufacturing and powder metallurgy, the primary methods for debinding are thermal, solvent, and catalytic debinding. Each technique is designed to remove the temporary "binder" material that holds metal or ceramic particles together, but they operate on different principles of chemistry and physics, offering distinct advantages in speed, cost, and applicability.
The core challenge of debinding is not simply removing the binder, but doing so without introducing stress, cracks, or distortion to the fragile part. Your choice of method is a critical trade-off between production speed, equipment cost, and the final integrity of your component.

Why Debinding is a Critical, Delicate Step
After initial forming, such as in Metal Injection Molding (MIM) or binder jetting, the component is in its "green state." It consists of fine powder particles held in shape by a polymer binder system.
This green part is weak and has no final material properties. Debinding is the intermediate step before sintering (where the powder is heated to fuse into a dense solid).
The goal is to create a network of open pores throughout the part, allowing the remaining binder to escape without building up internal pressure. If done improperly, the part can crack, blister, or warp, rendering it useless.
The Core Methods of Debinding
Each method targets different components within the binder system, which is often a carefully engineered mix of waxes and polymers.
Thermal Debinding: The Foundational Approach
Thermal debinding is the most straightforward method, relying solely on heat to remove the binder. The process occurs in a furnace with a precisely controlled atmosphere.
The part is heated slowly through several stages. At lower temperatures, waxes and low-molecular-weight polymers melt and evaporate. At higher temperatures, the remaining polymer backbone is removed through pyrolysis, or thermal decomposition.
This method is often the second stage in a two-part process (e.g., after solvent debinding) but can also be performed as a single, albeit very long, step.
Solvent Debinding: A Two-Stage Process
Solvent debinding removes a significant portion of the binder by immersing the green part in a liquid or vapor solvent. This solvent is chosen to dissolve one major component of the binder system (often a wax) while leaving another (the polymer "backbone") intact.
This process leaches out the soluble binder, creating an open-pore network throughout the part. The part, now in its "brown state," is more robust and ready for a final thermal debind and sintering.
Because it creates escape channels, this method is highly effective for parts with thick cross-sections, as it reduces the risk of defects during the final thermal stage.
Catalytic Debinding: The High-Speed Option
Catalytic debinding is a chemical process that uses a gaseous acid catalyst, typically nitric acid, to rapidly break down the primary binder.
This method requires a specific binder system, most commonly one based on polyoxymethylene (POM), also known as polyacetal. The catalyst triggers a chemical reaction that rapidly depolymerizes the POM into formaldehyde, which is then exhausted from the furnace.
It is the fastest of the three methods, reducing debinding times from many hours or days down to just a few hours.
Understanding the Trade-offs
The choice of debinding method is not arbitrary; it is determined by the feedstock material and production priorities.
Speed and Throughput
Catalytic debinding is by far the fastest, making it ideal for high-volume, automated production. Solvent debinding is significantly faster than a pure thermal process but slower than catalytic. Thermal-only debinding is the slowest method.
Equipment and Safety
Thermal debinding uses the simplest equipment—a furnace with atmospheric control. Solvent debinding requires equipment for handling and often recovering chemical solvents. Catalytic debinding requires the most specialized equipment, including corrosion-resistant furnaces and systems to safely handle gaseous acids.
Binder System Compatibility
The debinding method is inextricably linked to the binder used in the feedstock. You cannot use a catalytic process on a binder that isn't designed for it. The choice is often made when selecting the raw material for the process.
Part Geometry and Integrity
For parts with very thick cross-sections (>6-10mm), a two-stage solvent/thermal process is often preferred. The initial solvent stage effectively creates escape routes for gases, preventing the internal pressure buildup that can cause cracks during thermal removal.
Selecting the Right Debinding Strategy
Your decision should be based on a clear understanding of your operational priorities and the physical constraints of your parts.
- If your primary focus is maximum speed and high-volume production: Catalytic debinding is the industry standard, provided you use a compatible POM-based feedstock.
- If you are working with thick cross-sections or complex geometries: A two-stage solvent/thermal process offers a reliable balance of speed and reduced risk of part defects.
- If your primary focus is equipment simplicity or small-batch production: A well-controlled thermal debinding process is a viable and effective, though slower, solution.
Ultimately, the optimal debinding method aligns the characteristics of your part and production goals with the fundamental chemistry of binder removal.
Summary Table:
| Method | Key Principle | Speed | Best For |
|---|---|---|---|
| Thermal Debinding | Heat to melt/evaporate/pyrolyze binder | Slowest | Simplicity, small batches |
| Solvent Debinding | Dissolve binder component in liquid/vapor | Medium | Thick cross-sections, complex geometries |
| Catalytic Debinding | Gaseous acid catalyst breaks down binder | Fastest | High-volume, automated production |
Optimize Your Debinding Process with KINTEK
Choosing the right debinding method is critical for achieving high-quality, defect-free parts. KINTEK specializes in lab equipment and consumables, providing the precise furnaces and systems needed for reliable thermal and catalytic debinding processes.
Our expertise helps laboratories and manufacturers in additive manufacturing and powder metallurgy enhance production efficiency and ensure part integrity. Let us help you select the ideal equipment for your specific binder system and production goals.
Contact KINTEK today to discuss your debinding challenges and discover how our solutions can bring precision and reliability to your lab!
Visual Guide
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is the thermal debinding process? A Guide to Safe Binder Removal for MIM & Ceramics
- What is the purpose of a laboratory furnace? Achieve Precise High-Temperature Processing
- What is the annealing temperature of quartz? Achieve Optimal Thermal Stability for Your Components
- What are the disadvantages of dry ashing? Key Limitations for Accurate Elemental Analysis
- How do high-temperature furnaces and ceramic crucibles impact Li-ion battery stability? Master Precision Synthesis



















