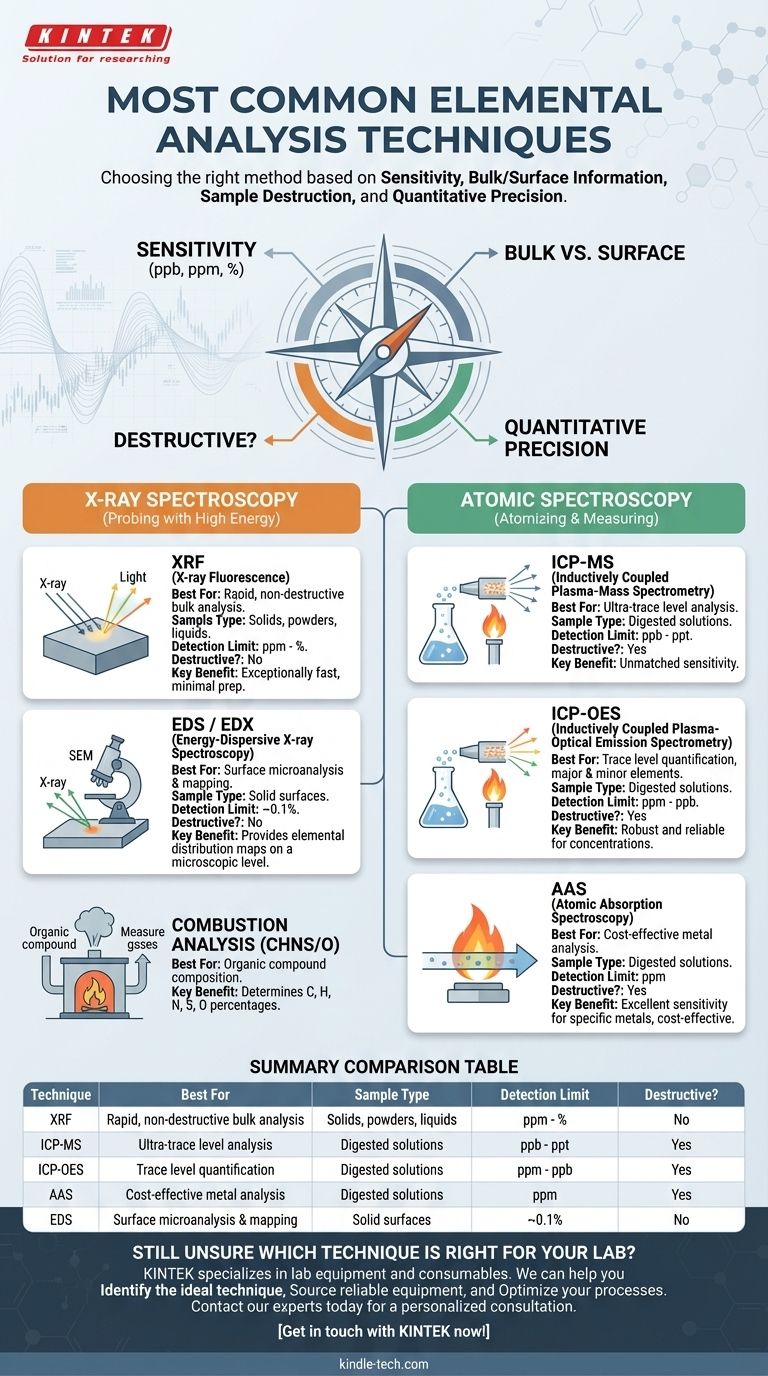
The most common elemental analysis techniques are X-ray Fluorescence (XRF), Inductively Coupled Plasma (ICP) based methods like ICP-Mass Spectrometry (ICP-MS) and ICP-Optical Emission Spectrometry (ICP-OES), Atomic Absorption Spectroscopy (AAS), and Energy-Dispersive X-ray Spectroscopy (EDS). These methods are widely used across industries from geology to manufacturing because they offer a range of capabilities for identifying and quantifying the elemental composition of a material.
The critical insight is not knowing what the techniques are, but understanding why you would choose one over another. Your choice will be dictated by a balance of four key factors: the required sensitivity, whether you need bulk or surface information, if the sample can be destroyed, and the need for quantitative precision.

The Core Categories of Analysis
Elemental analysis techniques operate on distinct physical principles. Understanding these principles is the first step in selecting the right tool for your specific analytical problem. We can group the most common methods into two primary families: those based on X-ray interactions and those based on atomic spectroscopy.
X-ray Spectroscopy: Probing with High Energy
These techniques use X-rays to excite atoms in a sample, causing them to emit characteristic secondary X-rays that act as elemental fingerprints.
X-ray Fluorescence (XRF) is a workhorse technique for bulk elemental analysis. It is exceptionally fast, requires minimal sample preparation, and is fundamentally non-destructive. It is ideal for analyzing solids, powders, and liquids, from magnesium (Mg) up through uranium (U) on the periodic table.
Energy-Dispersive X-ray Spectroscopy (EDS or EDX) is nearly always coupled with a Scanning Electron Microscope (SEM). While the principle is similar to XRF, EDS provides elemental information from a microscopic area on a sample's surface, making it a surface-sensitive microanalysis technique. It generates elemental maps that show the spatial distribution of elements.
Atomic Spectroscopy: Atomizing and Measuring
These techniques work by completely breaking a sample down into its constituent atoms, typically in a plasma or flame, and then measuring how those atoms interact with light. This process is inherently destructive.
Inductively Coupled Plasma (ICP) methods begin by digesting a sample into an acidic solution, which is then aerosolized into an extremely hot (around 10,000 K) argon plasma.
- ICP-Optical Emission Spectrometry (ICP-OES) measures the specific wavelengths of light emitted by the excited atoms in the plasma. It is robust and excellent for measuring concentrations down to the parts-per-million (ppm) level.
- ICP-Mass Spectrometry (ICP-MS) is a more sensitive evolution. Instead of measuring light, it funnels the ions from the plasma into a mass spectrometer to separate them by their mass-to-charge ratio. This allows for exceptional trace-level sensitivity, often reaching parts-per-billion (ppb) or even parts-per-trillion (ppt) detection limits.
Atomic Absorption Spectroscopy (AAS) is an older, but still highly relevant and cost-effective technique. It measures the amount of light absorbed by ground-state atoms in a flame. While it can typically only analyze one element at a time, it offers excellent sensitivity for specific metal analyses.
Combustion Analysis (CHNS/O) is a specialized technique for determining the elemental composition of organic compounds. The sample is combusted in a furnace, and the resulting gases (CO₂, H₂O, N₂, SO₂) are measured to determine the mass percent of carbon, hydrogen, nitrogen, and sulfur.
Key Decision Factors
Choosing the correct technique requires you to precisely define your analytical question. The "best" method is the one that provides the required data with the least amount of effort and cost.
Quantitative vs. Qualitative Results
Do you need to know what is there or exactly how much is there?
- Highly Quantitative: ICP-MS, ICP-OES, and AAS are the gold standards for accurate and precise quantitative results, assuming proper calibration.
- Semi-Quantitative: XRF and EDS are excellent for rapid identification and can provide good quantitative estimates, but typically do not match the precision of destructive atomic spectroscopy methods without extensive calibration.
Bulk vs. Surface Information
Are you interested in the overall composition of the material or just what is on its surface?
- Bulk Analysis: XRF (for solids) and all ICP/AAS methods (which analyze a digested, homogenized sample) provide the average bulk composition.
- Surface Analysis: EDS is the definitive choice for mapping elemental composition on a microscopic surface level.
Detection Limits and Sensitivity
How low of a concentration do you need to measure?
- Ultra-Trace Levels (ppb, ppt): ICP-MS is unmatched in its ability to detect elements at extremely low concentrations.
- Trace Levels (ppm): ICP-OES and AAS are excellent for measurements in the parts-per-million range.
- Major and Minor Elements (>0.1% to ppm): XRF is perfectly suited for measuring elements that are not at trace levels.
Understanding the Trade-offs
No technique is perfect. The primary trade-offs you will encounter are between speed, sensitivity, and sample preparation complexity.
The Speed vs. Precision Dilemma
XRF is incredibly fast, often providing a result in seconds to minutes with no sample destruction. However, its detection limits are higher than ICP methods. ICP-MS, on the other hand, offers unparalleled precision and sensitivity but requires a lengthy, destructive sample digestion process that can take hours.
Sample Preparation and Destruction
This is a critical logistical constraint. XRF stands out for its non-destructive nature, allowing a precious sample to be analyzed and then used for other purposes. All atomic spectroscopy techniques (ICP-MS, ICP-OES, AAS) are inherently destructive, as the sample must be dissolved in acid or combusted.
Cost and Accessibility
Cost is a major factor. A benchtop or handheld XRF is a relatively accessible instrument. AAS systems are also quite affordable. An ICP-MS system represents a significant capital investment and requires a dedicated, clean lab environment and a highly skilled operator.
Making the Right Choice for Your Goal
To select the right technique, start with your primary objective.
- If your primary focus is rapid, non-destructive screening of solid materials (e.g., alloy identification, RoHS compliance): XRF is the clear and immediate choice.
- If your primary focus is high-precision trace element analysis in environmental or biological samples: ICP-MS is the gold standard for its unmatched sensitivity.
- If your primary focus is routine, cost-effective quantification of a few specific metals in solution: AAS provides an excellent balance of performance and economy.
- If your primary focus is understanding elemental distribution on a microscopic surface (e.g., failure analysis, phase identification): SEM-EDS is the purpose-built tool for the job.
- If your primary focus is measuring major and minor elements in solutions with high accuracy: ICP-OES offers a robust and reliable solution that is less complex than ICP-MS.
Ultimately, the most effective analytical strategy begins with a clear definition of the problem you are trying to solve.
Summary Table:
| Technique | Best For | Sample Type | Detection Limit | Destructive? |
|---|---|---|---|---|
| XRF | Rapid, non-destructive bulk analysis | Solids, powders, liquids | ppm - % | No |
| ICP-MS | Ultra-trace level analysis | Digested solutions | ppb - ppt | Yes |
| ICP-OES | Trace level quantification | Digested solutions | ppm - ppb | Yes |
| AAS | Cost-effective metal analysis | Digested solutions | ppm | Yes |
| EDS | Surface microanalysis & mapping | Solid surfaces | ~0.1% | No |
Still Unsure Which Technique is Right for Your Lab?
Choosing the correct elemental analysis method is critical for accurate results and efficient workflows. The team at KINTEK specializes in lab equipment and consumables, serving diverse laboratory needs across research, quality control, and manufacturing.
We can help you:
- Identify the ideal technique (XRF, ICP-MS, ICP-OES, AAS, EDS) based on your specific samples and sensitivity requirements.
- Source reliable equipment from leading manufacturers to ensure precision and durability.
- Optimize your analytical processes with expert support and high-quality consumables.
Don't let analytical uncertainty slow down your research or production. Contact our experts today for a personalized consultation and let us help you achieve precise and reliable elemental analysis.
Visual Guide
Related Products
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Platinum Auxiliary Electrode for Laboratory Use
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Proton Exchange Membrane for Batteries Lab Applications
- Oxygen Probe to Measure Temperature and Active Oxygen Content in Molten Steel
People Also Ask
- What does sintering decrease? Mastering Porosity, Surface Area, and Material Properties
- How efficient is plastic pyrolysis? Maximizing Waste-to-Energy Conversion
- What is the function of a magnetic stirrer in sol-gel catalyst synthesis? Ensure Perfect Zeolite-Titanate Uniformity
- What is the flow rate of a filter press? Mastering the Dynamic Filtration Cycle
- What are the two common orientations of Ultra Freezers? Upright vs. Chest for Your Lab
- What is called sputtering? The Ultimate Guide to High-Quality Thin Film Deposition
- What are the defects of sintered metal? Understanding Porosity, Cracking & Distortion
- What is the cost of biomass production? Unpacking the True 'Delivered Cost' to Your Facility
















