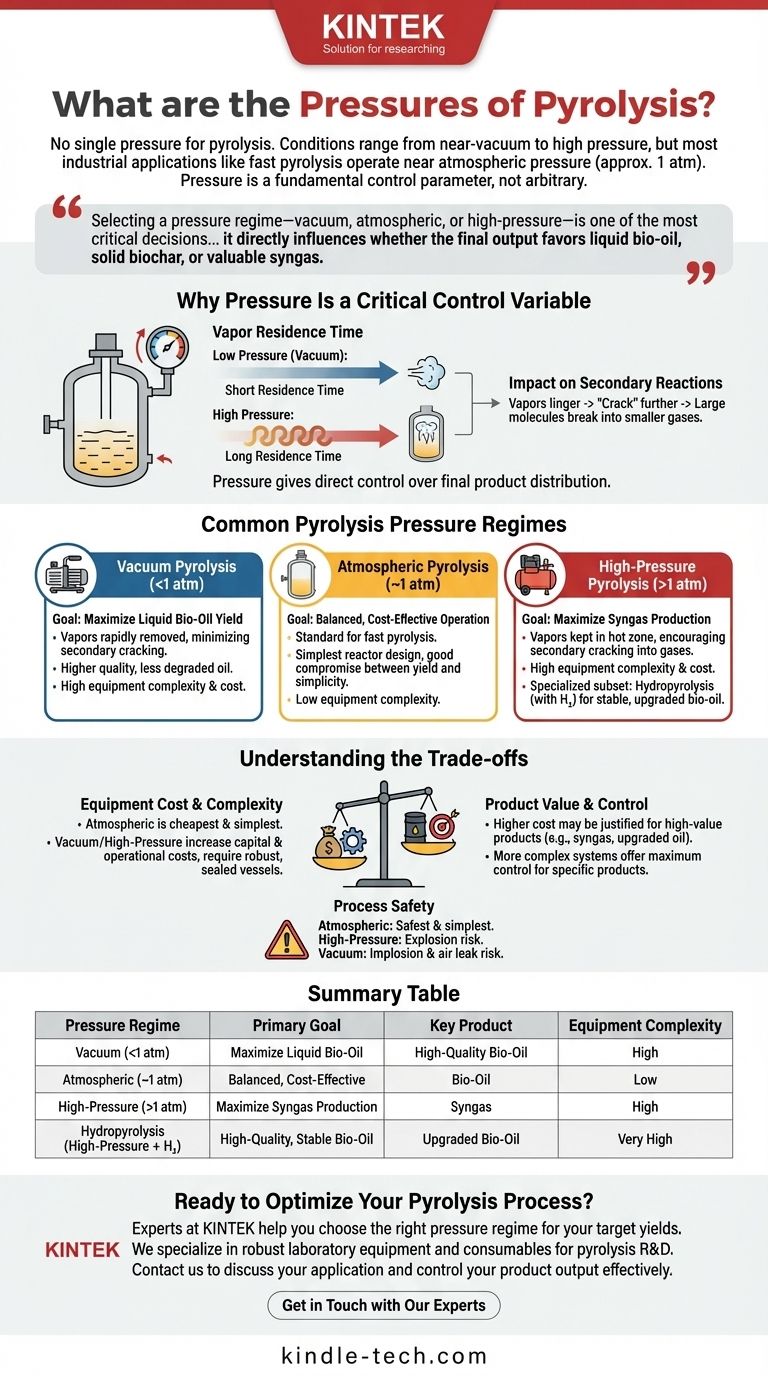
In short, there is no single pressure for pyrolysis. The process can be run under a wide range of conditions, from a near-perfect vacuum to extremely high pressures. However, the vast majority of common industrial applications, such as fast pyrolysis for bio-oil production, operate at or very near standard atmospheric pressure (approximately 1 atm or 101.3 kPa).
The choice of pressure is not arbitrary; it is a fundamental control parameter. Selecting a pressure regime—vacuum, atmospheric, or high-pressure—is one of the most critical decisions in designing a pyrolysis process, as it directly influences whether the final output favors liquid bio-oil, solid biochar, or valuable syngas.

Why Pressure Is a Critical Control Variable
Understanding pyrolysis requires seeing pressure as a lever that steers the chemical reactions. Its primary influence is on the residence time of hot gases within the reactor.
The Role of Vapor Residence Time
As the feedstock (like biomass or plastic) heats up, it decomposes and releases volatile organic vapors. The pressure inside the reactor determines how quickly these vapors can escape.
At low pressures (vacuum), there is a strong driving force pulling these vapors out of the hot zone almost instantly.
At high pressures, these vapors are compressed and forced to remain in the hot reactor for a longer period.
Impact on Secondary Reactions
This residence time is critical because it governs secondary reactions. When the initial vapors linger in the hot zone, they "crack" further, breaking down from large, condensable molecules (which form liquid oil) into smaller, non-condensable gas molecules (like hydrogen, methane, and carbon monoxide).
Therefore, pressure gives you direct control over the final product distribution.
Common Pyrolysis Pressure Regimes
Engineers choose a pressure regime based on the desired end product. Each has a distinct purpose and equipment profile.
Vacuum Pyrolysis (<1 atm)
The goal here is to maximize liquid bio-oil yield. By operating under a vacuum, volatile vapors are rapidly removed from the reactor before they have a chance to undergo secondary cracking into gases.
This produces a higher quality, less degraded oil but requires more complex and expensive vacuum-sealed reactors and pumping systems.
Atmospheric Pyrolysis (~1 atm)
This is the most common and economically balanced approach. It is the standard for fast pyrolysis, a technique designed to produce high yields of bio-oil.
Operating at atmospheric pressure simplifies reactor design significantly, avoiding the high costs and engineering challenges of both vacuum and high-pressure systems. It offers a good compromise between liquid yield and operational simplicity.
High-Pressure Pyrolysis (>1 atm)
The primary goal of high-pressure pyrolysis is to maximize the yield of syngas. By keeping the vapors in the hot zone under pressure, secondary cracking is encouraged, converting potential oils into a gas mixture.
A specialized subset is hydropyrolysis, where pyrolysis occurs under high pressure in a hydrogen-rich atmosphere. This produces a more stable, higher-quality bio-oil with fewer oxygenates, but at a substantially higher equipment and operational cost.
Understanding the Trade-offs
Choosing a pressure regime involves balancing competing factors. There is no single "best" pressure, only the most appropriate one for a specific technical and economic goal.
Equipment Cost vs. Product Value
Atmospheric systems are the cheapest to build and operate. Vacuum and high-pressure systems require robust, perfectly sealed vessels and ancillary equipment (pumps, compressors) that dramatically increase capital and operational expenses.
This higher cost may be justified if the end product (e.g., high-quality syngas or stabilized oil from hydropyrolysis) has a higher market value than standard bio-oil.
Process Safety and Simplicity
Atmospheric pressure is inherently the safest and simplest condition. High-pressure systems carry a risk of explosive failure due to the high amount of stored energy. Vacuum systems carry an implosion risk and are highly sensitive to air leaks, which can create an explosive atmosphere inside the reactor.
Product Distribution Control
This is the central trade-off. If you want maximum control to produce a specific product—either pure liquids or pure gases—you will likely need to invest in a more complex vacuum or high-pressure system. If a balanced output is acceptable, atmospheric pressure is sufficient.
Making the Right Choice for Your Goal
Your decision should be dictated entirely by your primary objective.
- If your primary focus is maximizing liquid bio-oil yield: You should use vacuum pyrolysis to minimize secondary cracking of valuable vapors.
- If your primary focus is a balanced, cost-effective operation: You should use atmospheric pyrolysis, which offers a good compromise on yield and requires the simplest equipment.
- If your primary focus is producing syngas for fuel or chemical synthesis: You should use high-pressure pyrolysis to intentionally promote the secondary cracking of vapors into gas molecules.
- If your primary focus is producing a higher-grade, more stable liquid fuel: You should investigate hydropyrolysis, a specialized high-pressure technique.
By understanding its effects, you transform pressure from a simple setting into a precise tool for targeted chemical production.
Summary Table:
| Pressure Regime | Primary Goal | Key Product | Equipment Complexity |
|---|---|---|---|
| Vacuum (<1 atm) | Maximize Liquid Bio-Oil | High-Quality Bio-Oil | High (Sealed Reactors, Pumps) |
| Atmospheric (~1 atm) | Balanced, Cost-Effective Operation | Bio-Oil | Low (Simplest Design) |
| High-Pressure (>1 atm) | Maximize Syngas Production | Syngas | High (Robust, Sealed Vessels) |
| Hydropyrolysis (High-Pressure + H₂) | High-Quality, Stable Bio-Oil | Upgraded Bio-Oil | Very High (Specialized) |
Ready to Optimize Your Pyrolysis Process?
Choosing the right pressure regime is critical for achieving your target product yields of bio-oil, biochar, or syngas. The experts at KINTEK are here to help.
We specialize in providing robust laboratory equipment and consumables for pyrolysis research and development. Whether you are scaling a process or optimizing reactions in the lab, our solutions are designed for precision and reliability.
Contact us today to discuss how we can support your specific pyrolysis application and help you control your product output effectively.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- What roles do autoclaves play in MFI zeolite synthesis? Master Hydrothermal Crystalline Growth
- How does a high-pressure reactor demonstrate its value in accelerated aging? Predict Catalyst Durability Fast
- What is the purpose of using a high-temperature hydrothermal reactor? Enhance Iodine@Activated Carbon Cathode Synthesis
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield



















