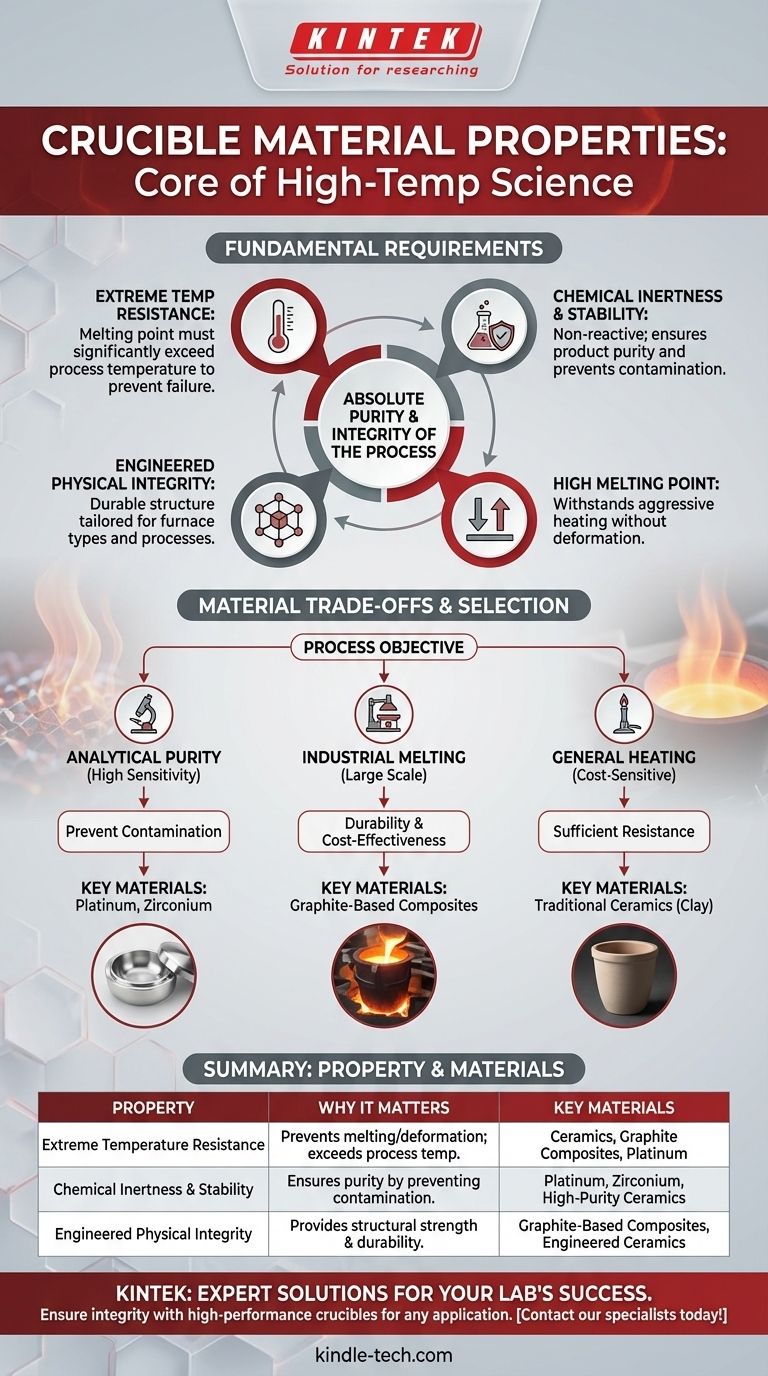
A crucible's value is defined by four core properties: extreme temperature resistance, chemical inertness, physical stability, and a melting point significantly higher than the material it holds. These characteristics ensure the crucible can contain and withstand aggressive, high-temperature processes without failing or contaminating the contents.
The central purpose of a crucible is to act as a completely neutral vessel in an extreme environment. Its material properties are not just about survival; they are about ensuring the absolute purity and integrity of the process it contains.

The Fundamental Requirements of a Crucible
To function correctly, any crucible material must satisfy a set of non-negotiable criteria. These properties are the foundation upon which all high-temperature chemistry and metallurgy are built.
Extreme Temperature Resistance
The most obvious requirement is the ability to withstand heat. A crucible's melting point must be substantially higher than the working temperature of the material inside it.
This prevents the crucible itself from melting, deforming, or failing structurally during the heating process, which would result in a catastrophic loss of containment.
Chemical Inertness and Stability
A crucible must be chemically compatible with the molten materials it holds. It cannot react with, dissolve in, or otherwise contaminate the melt.
This chemical stability is critical for two reasons. First, it prevents the crucible from deteriorating and weakening. Second, and more importantly, it ensures the purity of the final product, which is paramount in applications like analytical chemistry and the creation of high-purity alloys.
Engineered Physical Integrity
Modern crucibles are often not simple vessels but highly engineered composite materials. Their performance depends heavily on their physical structure.
For example, many contemporary crucibles are graphite-based composites. The precise composition and the structural alignment of the graphite are controlled to optimize performance for specific furnace types, whether fuel-fired, electric resistance, or induction.
Understanding Material Trade-offs
No single crucible material is perfect for every application. The choice always involves a balance between performance, cost, and the specific demands of the process.
Historical vs. Modern Materials
Historically, crucibles were made from simple materials like clay, which offered sufficient heat resistance for ancient metallurgy.
Today, materials range from these traditional ceramics to advanced composites and pure metals like platinum or zirconium. This heterogeneity reflects the diverse and demanding applications of modern science and industry.
Purity vs. Cost
For highly sensitive tasks like analytical sample preparation, preventing any contamination is the top priority. In these cases, extremely inert but expensive materials like platinum are used.
For large-scale industrial melting where tons of metal are processed, a minor level of contamination may be acceptable. Here, more cost-effective and durable graphite-based composites are the standard choice.
Application-Specific Design
The shape, size, and material of a crucible are dictated by its use. A small cup for a laboratory test has different requirements than a massive, multi-ton vessel used in a foundry.
Furthermore, the material must be compatible with the heating method. The properties needed for an induction furnace are different from those required for a fuel-fired furnace, influencing the final material selection.
Making the Right Choice for Your Goal
Selecting the correct crucible material is fundamental to the success of your work. Your final choice should be guided by the primary objective of your process.
- If your primary focus is analytical purity: Choose a highly inert material like platinum or zirconium to eliminate the risk of sample contamination.
- If your primary focus is industrial-scale melting: Select an engineered, graphite-based composite designed for durability and compatibility with your specific furnace type.
- If your primary focus is general, cost-sensitive heating: Traditional ceramic materials like clay can be a suitable option for applications where absolute purity is not the main concern.
Understanding these core material properties empowers you to control your high-temperature environment with precision and confidence.
Summary Table:
| Property | Why It Matters | Key Materials |
|---|---|---|
| Extreme Temperature Resistance | Prevents melting/deformation; melting point must exceed process temperature. | Ceramics, Graphite Composites, Platinum |
| Chemical Inertness & Stability | Ensures product purity by preventing contamination from the crucible. | Platinum, Zirconium, High-Purity Ceramics |
| Engineered Physical Integrity | Provides structural strength and durability for specific furnace types and processes. | Graphite-Based Composites, Engineered Ceramics |
Selecting the right crucible is critical to your lab's success. The material properties directly impact the purity of your results and the efficiency of your processes. KINTEK specializes in lab equipment and consumables, offering a range of high-performance crucibles designed for analytical purity, industrial-scale melting, and cost-sensitive applications. Our experts can help you choose the perfect crucible material for your specific furnace type and process goals. Ensure the integrity of your high-temperature work—contact our specialists today for a personalized consultation!
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- Why is a corundum crucible preferred for high-purity magnesium? Achieve 99.999% Purity Without Contamination
- Do crucibles break easily? Understanding Thermal Shock and Proper Handling
- Why are alumina crucibles used for LLZO calcination? Optimize Cubic Phase Stability and Thermal Resilience
- What is the function of a tantalum-lined fused silica ampoule? Ensure High-Purity LBE Sample Preparation
- Why is porcelain used for crucible? Discover the Ideal Balance of Heat Resistance and Affordability
- What are the different sizes of crucibles? A Guide from Jewelry to Industrial Scales
- What temperature does crucible melt? Choose the Right Material for Your High-Temp Needs
- What is a quartz crucible? The Essential Vessel for High-Purity Silicon Crystal Growth



















