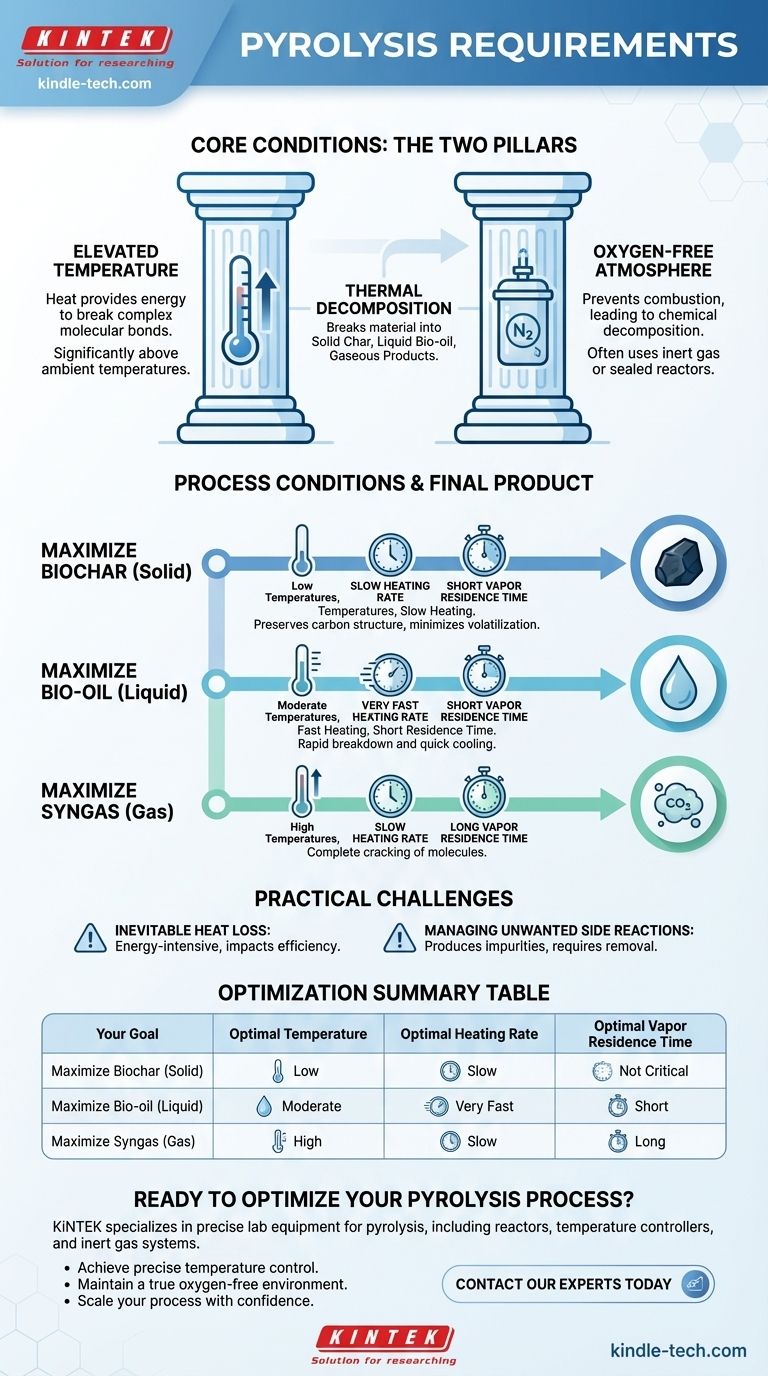
At its core, pyrolysis requires two fundamental conditions. The process involves the thermal decomposition of materials at high temperatures, but critically, this must occur in an oxygen-deficient or completely oxygen-free environment. This absence of oxygen is what prevents combustion and instead causes the material's chemical bonds to break down into a mix of solid, liquid, and gaseous products.
The specific requirements for temperature, heating rate, and vapor residence time are not universal; they are strategic variables you must adjust to control whether the final output is predominantly solid char, liquid bio-oil, or combustible gas.

The Two Foundational Pillars of Pyrolysis
Pyrolysis is a controlled chemical breakdown, not a simple burning process. To achieve this, two non-negotiable environmental conditions must be met.
Requirement 1: Elevated Temperature
Heat is the engine of pyrolysis. It provides the necessary energy to break the complex molecular bonds within the feedstock.
The required temperature range is wide and depends heavily on the feedstock and desired products, but it is always significantly above ambient temperatures.
Requirement 2: An Oxygen-Free Atmosphere
This is the defining requirement that distinguishes pyrolysis from combustion. By removing oxygen, you prevent the material from burning and releasing its energy primarily as heat.
Instead of combusting, the material decomposes into valuable chemical components. This is typically achieved by using an inert gas like nitrogen or by designing a sealed reactor where incoming oxygen is eliminated.
How Process Conditions Dictate the Final Product
Once the two foundational requirements are met, you can manipulate several process variables to steer the chemical reactions toward a specific output. The balance between these variables determines the final yield of solids, liquids, and gases.
Optimizing for Biochar (Solid)
To maximize the yield of solid char, the goal is a slow, controlled breakdown that preserves the carbon structure.
This is achieved with low temperatures and slow heating rates. This gentle process minimizes the volatilization of the material, leaving behind a carbon-rich solid.
Optimizing for Bio-oil (Liquid)
To produce the highest yield of liquid products (bio-oil), the process must rapidly break down the feedstock and immediately cool the resulting vapors.
This requires moderate temperatures, but with very high heating rates and a short gas residence time. The vapors are quickly removed from the hot zone and condensed into a liquid before they can break down further into gas.
Optimizing for Syngas (Gas)
If the goal is to maximize gaseous products, the process must be intense enough to break down not only the original feedstock but also the intermediate liquid vapors.
This is best accomplished with high temperatures, low heating rates, and a long gas residence time. This allows for the complete "cracking" of larger hydrocarbon molecules into smaller, non-condensable gas molecules like hydrogen and methane.
Understanding the Practical Challenges
Achieving the ideal conditions in practice involves significant engineering challenges and trade-offs that impact efficiency and product purity.
Inevitable Heat Loss
Pyrolysis is an energy-intensive process. Maintaining high temperatures in a reactor means that process-specific heat loss is a major factor in overall energy efficiency. Any energy that escapes to the environment is wasted and increases operational costs.
Managing Unwanted Side Reactions
The chemical environment inside a pyrolysis reactor is complex. Side reactions are common and can produce undesirable byproducts, such as complex aromatic compounds or various hydrocarbons.
If the goal is a pure product, like industrial-grade hydrogen from methane pyrolysis, these impurities must be accounted for and removed, adding complexity and cost to the process.
Making the Right Choice for Your Goal
The optimal requirements for your pyrolysis process depend entirely on the product you value most.
- If your primary focus is maximizing solid char: Utilize low temperatures and slow heating rates to favor a controlled, solid-state conversion.
- If your primary focus is producing liquid bio-oil: Employ moderate temperatures with fast heating rates and ensure vapors are removed and condensed quickly.
- If your primary focus is generating combustible gas: Apply high temperatures and allow the vapors a long residence time in the hot zone to ensure they fully break down.
Ultimately, mastering pyrolysis is about precisely controlling its core conditions to dictate the outcome of the chemical transformation.
Summary Table:
| Your Goal | Optimal Temperature | Optimal Heating Rate | Optimal Vapor Residence Time |
|---|---|---|---|
| Maximize Biochar (Solid) | Low | Slow | Not Critical |
| Maximize Bio-oil (Liquid) | Moderate | Very Fast | Short |
| Maximize Syngas (Gas) | High | Slow | Long |
Ready to build or optimize your pyrolysis process?
At KINTEK, we specialize in the precise lab equipment and consumables needed to master pyrolysis. Whether you're researching feedstock conversion, optimizing for bio-oil yield, or scaling up syngas production, our reactors, temperature controllers, and inert gas systems are engineered for accuracy and reliability.
We provide the tools to help you:
- Achieve precise temperature control for consistent results.
- Maintain a true oxygen-free environment to prevent combustion.
- Scale your process from R&D to pilot plant with confidence.
Let's discuss your project requirements. Contact our experts today to find the right solution for your laboratory's needs.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- What are the typical heating zone configurations and maximum temperature capabilities of tube furnaces? Find the Right Setup for Your Lab
- How do a quartz tube reactor and atmosphere furnace collaborate in Co@NC pyrolysis? Master Precision Synthesis



















