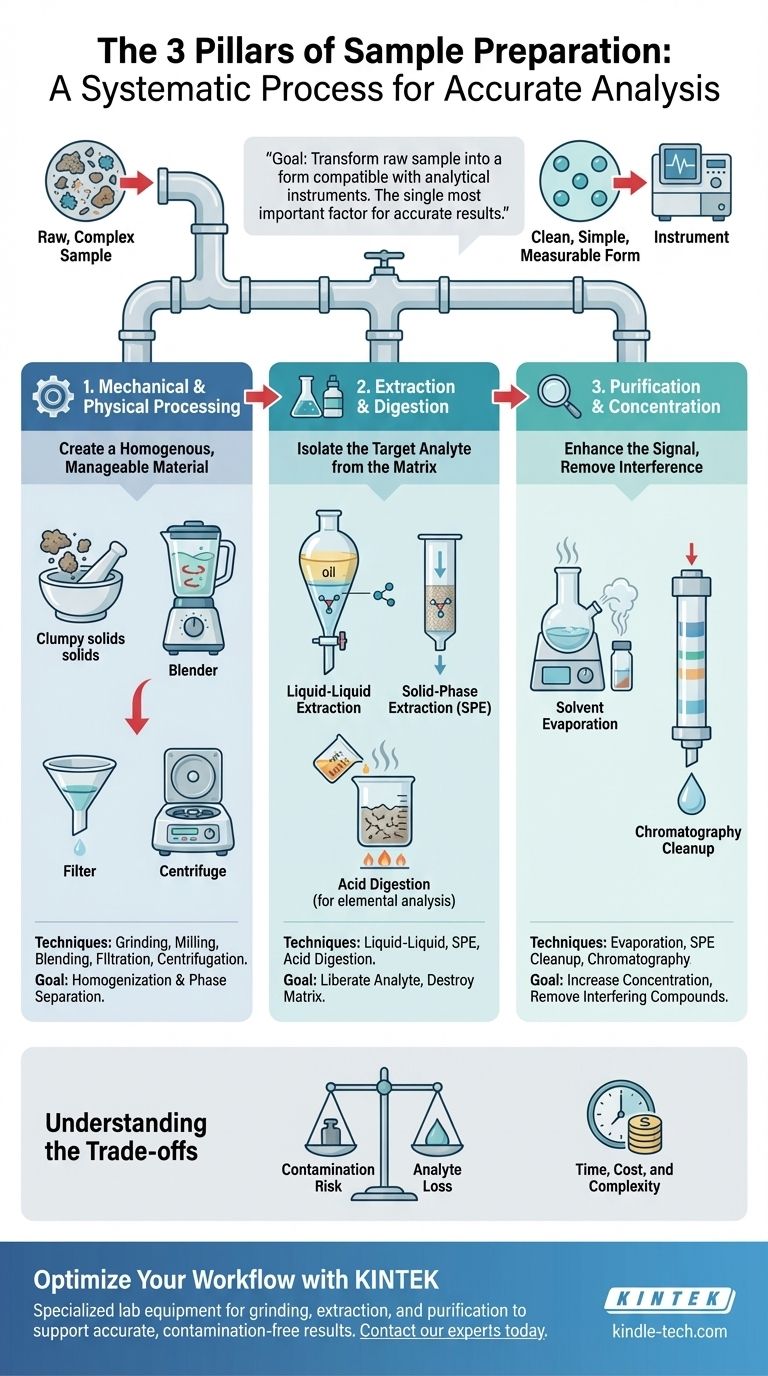
While there are hundreds of specific methods, sample preparation is not about choosing one of three specific techniques. Instead, it's about a systematic process that can be understood through three fundamental categories of action: mechanical processing, chemical extraction/digestion, and purification/concentration. These stages ensure your sample is uniform, your target analyte is accessible, and interfering substances are removed.
The goal of sample preparation is to transform a raw, complex sample into a clean, simple, and measurable form that is compatible with your analytical instrument. Getting this step right is the single most important factor in achieving accurate and reliable results.

The Foundation: Mechanical & Physical Processing
The first step in any analysis is to address the physical nature of the sample. The goal here is to create a homogenous and manageable material that accurately represents the original bulk substance.
Why Homogenization is Critical
A homogenous sample ensures that any small portion you take for analysis is identical to any other portion. Without this, your results will be inconsistent and unreliable.
For solid samples, this is often achieved through grinding, milling, or crushing. For liquid or semi-solid samples like tissues or wastewater, techniques like blending or sonication are used to create a uniform mixture.
The Role of Phase Separation
Many samples are mixtures of solids, liquids, and gases. Before you can analyze your target, you often need to separate these phases.
Simple techniques like filtration remove solid particles from a liquid, while centrifugation uses rotational force to separate substances based on density, such as pelleting cells from a culture medium.
Isolating the Target: Extraction & Digestion
Once the sample is physically uniform, the next challenge is to liberate the specific molecule or element of interest—the analyte—from the complex sample matrix.
Releasing the Analyte with Extraction
Extraction uses a solvent to selectively dissolve the analyte, leaving behind unwanted material. This is one of the most common preparation strategies.
Liquid-liquid extraction uses two immiscible liquids (like oil and water) to separate compounds based on their relative solubility. Solid-phase extraction (SPE) is a more advanced technique where the sample is passed through a solid material (a sorbent) that selectively traps the analyte, which can then be washed off and collected in a clean solvent.
Breaking It All Down with Digestion
For elemental analysis (e.g., measuring heavy metals), the complex organic matrix must be completely destroyed to free the atoms for measurement.
This is typically done using acid digestion, where strong acids and high temperatures are used to break down all organic components, leaving only the inorganic elements in a simple, clean liquid solution.
Enhancing the Signal: Purification & Concentration
The final stage addresses two key problems: low analyte levels and the presence of interfering substances. The goal is to produce a clean, concentrated sample that gives a strong, unambiguous signal in the analytical instrument.
Increasing Analyte Concentration
If your analyte is present in trace amounts, you may need to concentrate it before it can be detected.
A common method is solvent evaporation, where the sample is gently heated under vacuum or a stream of nitrogen to remove excess solvent, thereby increasing the analyte's concentration.
Removing Interference
Interfering compounds in the sample matrix can obscure the analyte's signal, leading to inaccurate results. These must be removed in a "cleanup" step.
Techniques like the previously mentioned SPE are excellent for cleanup. Similarly, various forms of chromatography can be used to separate the analyte from closely related interfering compounds before the final analysis.
Understanding the Trade-offs
No sample preparation method is perfect. The choice of technique always involves balancing competing factors, and being aware of these trade-offs is crucial for developing a robust method.
Risk of Contamination
Every step—every tool, every solvent, every transfer—introduces a risk of contaminating your sample with outside substances, which can lead to falsely high results.
Analyte Loss
Conversely, with every transfer, filtration, or extraction, there is a risk of losing a portion of your analyte, which can lead to falsely low results. The goal is to maximize recovery while minimizing contamination.
Time, Cost, and Complexity
A simple "dilute-and-shoot" method is fast and cheap but only works for the simplest samples. Complex, multi-step procedures using techniques like SPE provide cleaner samples and better data but are significantly more time-consuming and expensive.
Making the Right Choice for Your Goal
The ideal sample preparation workflow depends entirely on your sample type, your target analyte, and the sensitivity required by your analytical instrument.
- If your primary focus is elemental analysis (e.g., metals in soil): Your workflow will almost certainly involve mechanical grinding followed by a strong acid digestion.
- If your primary focus is quantifying an organic compound (e.g., a pesticide in water): Your strategy will likely involve filtration, followed by a liquid-liquid or solid-phase extraction and possible concentration.
- If your primary focus is analyzing a protein in biological tissue: You will need a homogenization step, followed by centrifugation, and likely some form of chromatographic cleanup to isolate the protein from a complex biological matrix.
Ultimately, designing an effective sample preparation strategy is the most critical and intellectually demanding part of chemical analysis.
Summary Table:
| Stage | Goal | Common Techniques |
|---|---|---|
| Mechanical & Physical Processing | Create a homogenous, representative sample | Grinding, milling, blending, filtration, centrifugation |
| Extraction & Digestion | Isolate the target analyte from the sample matrix | Liquid-liquid extraction, solid-phase extraction (SPE), acid digestion |
| Purification & Concentration | Remove interference and increase analyte concentration | Solvent evaporation, SPE, chromatography |
Ready to optimize your sample preparation workflow? The right lab equipment is fundamental to achieving accurate, contamination-free results. KINTEK specializes in high-quality lab equipment and consumables—from mills and homogenizers to extraction and purification systems—to support your laboratory's specific needs. Contact our experts today to discuss how we can help you enhance your analytical accuracy and efficiency.
Visual Guide
Related Products
- Three-dimensional electromagnetic sieving instrument
- Laboratory Single Horizontal Jar Mill
- Laboratory Test Sieves and Sieving Machines
- Mini Planetary Ball Mill Machine for Laboratory Milling
- High Energy Planetary Ball Mill Milling Machine for Laboratory
People Also Ask
- What are the types of sintering? A Guide to Solid-State, Liquid-Phase, and Reactive Methods
- What are the functions of a laboratory stirring system in enhancing the leaching efficiency of gold scrap?
- Why are ultra-low temperature freezers important in scientific research? Ensure Sample Integrity and Reproducibility
- What is the range of RF sputtering? Expanding Your Thin Film Capabilities Beyond Metals
- What is the primary function of a vacuum pump? Remove Gas Molecules to Create a Controlled Vacuum
- What is the product composition of pyrolysis gas? A Guide to Fuel Composition & Control
- What is the difference between pyrolysis and catalytic cracking? A Guide to Process Selection
- Why DC sputtering is not used for insulators? Overcome the Charge-Up Effect with RF Sputtering



















