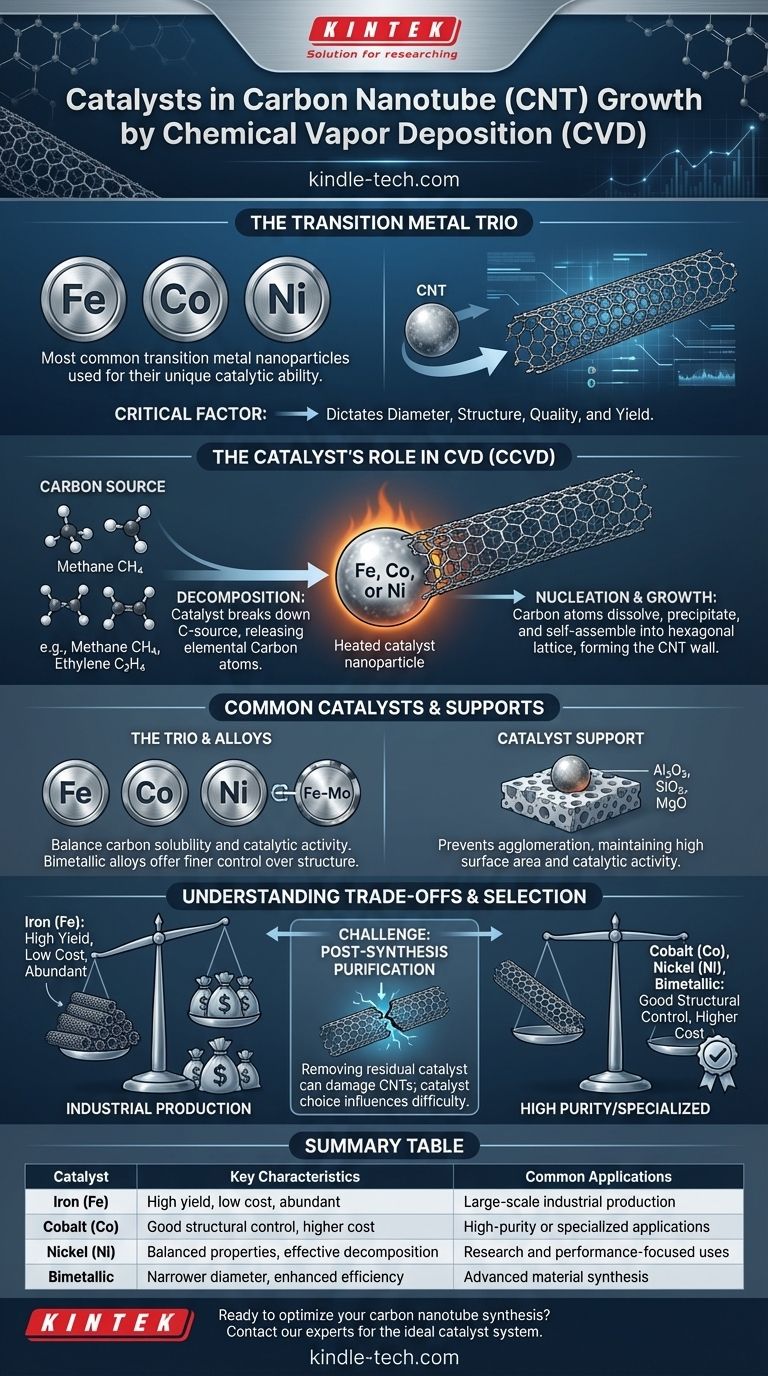
The most common catalysts used for growing carbon nanotubes (CNTs) via Chemical Vapor Deposition (CVD) are nanoparticles of transition metals. Specifically, Iron (Fe), Cobalt (Co), and Nickel (Ni) are the most widely employed due to their unique ability to decompose carbon-containing gases and nucleate the growth of the nanotube structure.
The choice of catalyst is not merely an ingredient in the process; it is the single most critical factor that dictates the resulting CNTs' diameter, structure, quality, and yield. Understanding the catalyst's function is fundamental to controlling the entire synthesis.

The Catalyst's Role in CNT Growth
To understand why specific metals are used, it's essential to understand the two critical functions they perform during the CVD process. This method is often called Catalytic Chemical Vapor Deposition (CCVD) because the catalyst is non-negotiable for success.
Carbon Source Decomposition
First, the heated catalyst nanoparticle acts as a site for breaking down the carbon-source gas (such as acetylene, ethylene, or methane). The metal surface has a high catalytic activity that efficiently breaks the chemical bonds of the hydrocarbon molecules, releasing elemental carbon atoms.
Nucleation and Growth
Once freed, these carbon atoms dissolve into and diffuse through the metal nanoparticle. When the metal becomes supersaturated with carbon, the carbon precipitates out onto the surface. This precipitated carbon self-assembles into the hexagonal lattice structure that forms the wall of the carbon nanotube, which then continues to grow from the catalyst particle.
Common Catalysts and Support Materials
While a few metals dominate the field, they are rarely used in their bulk form. Instead, they are prepared as nanoparticles and often stabilized on a secondary material known as a support.
The Transition Metal Trio: Fe, Co, Ni
Iron (Fe), Cobalt (Co), and Nickel (Ni) are uniquely suited for CNT growth because they have the right balance of properties. They possess good carbon solubility at typical CVD temperatures and exhibit the high catalytic activity needed to decompose hydrocarbons effectively.
The Importance of a Catalyst Support
The metal catalyst nanoparticles are typically deposited onto a stable, high-surface-area support material. This support prevents the nanoparticles from agglomerating at high temperatures, which would kill their catalytic activity. Common supports include alumina (Al₂O₃), silica (SiO₂), and magnesium oxide (MgO).
Bimetallic and Alloy Catalysts
To gain finer control over the CNT structure or improve growth efficiency, researchers often use bimetallic catalysts. For example, an Fe-Mo alloy can sometimes produce CNTs with a narrower diameter distribution or higher yield compared to using iron alone.
Understanding the Trade-offs
The selection of a catalyst system involves balancing performance, cost, and the complexity of post-processing. There is no single "best" catalyst for all applications.
Catalyst Purity and CNT Quality
The purity of the final CNT product is directly linked to the catalyst. After synthesis, the metal nanoparticles remain, often encapsulated at the tips or embedded within the walls of the nanotubes. These metallic impurities can be detrimental to the electronic and mechanical properties of the final product.
The Challenge of Post-Synthesis Purification
Removing the residual catalyst is a mandatory but often aggressive step. It typically involves strong acid treatments that can damage the structure of the CNTs, introducing defects into their walls and shortening their length. The choice of catalyst can influence how difficult this purification step will be.
Cost vs. Performance
As noted, cost-effectiveness is a major driver in CNT synthesis. Iron is by far the cheapest and most abundant catalyst, making it the preferred choice for large-scale industrial production where bulk yield is the primary goal. Cobalt and Nickel are more expensive but can offer better control over diameter and structure in certain research or high-performance applications.
Making the Right Choice for Your Goal
The optimal catalyst system is defined by your ultimate objective. By understanding the interplay between the catalyst, the support, and the growth conditions, you can tailor the synthesis to your specific needs.
- If your primary focus is high-yield, low-cost production: An iron (Fe) catalyst deposited on an alumina (Al₂O₃) support is the industry standard.
- If your primary focus is high structural quality and purity: A cobalt (Co) or bimetallic catalyst system may provide better control, despite higher costs and potentially complex purification.
- If your primary focus is specific electronic properties: The catalyst choice is critical, as it directly influences the diameter and chirality of the CNTs, which in turn determine if they are metallic or semiconducting.
Ultimately, the catalyst nanoparticle is the template from which the remarkable structure of a carbon nanotube originates.
Summary Table:
| Catalyst | Key Characteristics | Common Applications |
|---|---|---|
| Iron (Fe) | High yield, low cost, abundant | Large-scale industrial production |
| Cobalt (Co) | Good structural control, higher cost | High-purity or specialized applications |
| Nickel (Ni) | Balanced properties, effective carbon decomposition | Research and performance-focused uses |
| Bimetallic (e.g., Fe-Mo) | Narrower diameter distribution, enhanced efficiency | Advanced material synthesis |
Ready to optimize your carbon nanotube synthesis? The right catalyst is crucial for achieving the desired CNT diameter, structure, and purity. At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored for advanced material research, including CVD processes. Our expertise can help you select the ideal catalyst system for your specific goals, whether you're focused on high yield, superior quality, or specific electronic properties. Contact our experts today to discuss how we can support your laboratory's innovation and efficiency in CNT growth and beyond.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the resistance of CVD graphene? Achieve Superior Conductivity and Transparency
- What is the effect of temperature on graphene oxide? Master Thermal Reduction for Precise Material Properties
- What is sol gel method for thin films? A Low-Cost Path to High-Purity Coatings
- What are the advantages of diamond coating? Boost Durability and Performance
- Is diamond coating worth it? Maximize Component Life and Performance
- What are the growth processes of thin films? Master the 3 Modes for Precise Material Engineering
- What is the synthesis of graphene by chemical vapor deposition? Scalable Production of High-Quality Films
- What does a sputtering target do? It's the High-Purity Source for Precision Thin Films



















