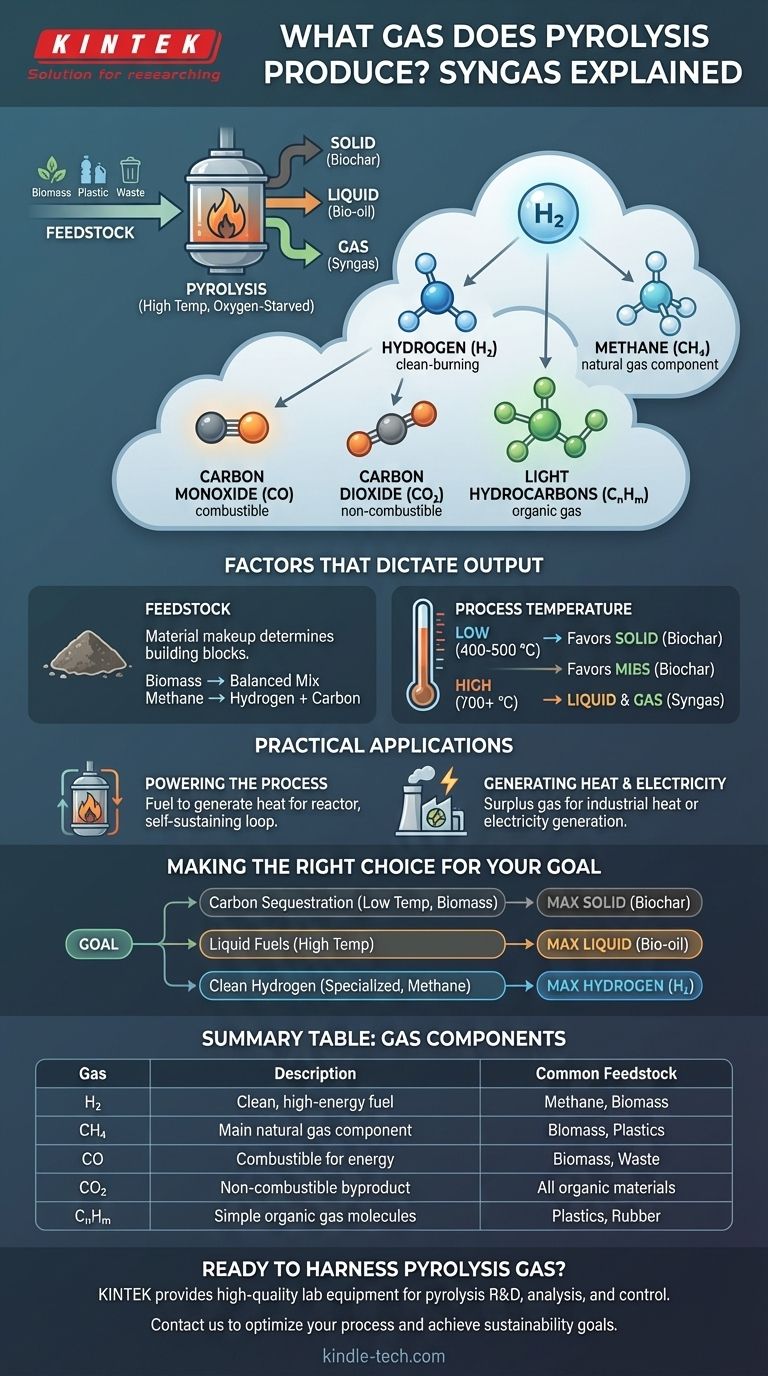
In short, pyrolysis produces a mixture of combustible gases, often called "syngas" or "pyrolysis gas." This is not a single gas but a blend primarily composed of hydrogen (H2), methane (CH4), carbon monoxide (CO), carbon dioxide (CO2), and various other light hydrocarbons (CnHm). The exact composition is highly dependent on the material being processed and the specific conditions of the pyrolysis reaction.
The critical takeaway is that pyrolysis doesn't create one specific gas. Instead, it generates a variable fuel gas mixture whose composition—and therefore its value and utility—is determined by two key factors: the initial material (feedstock) and the process temperature.

Deconstructing Pyrolysis Gas
Pyrolysis is the thermal decomposition of materials at high temperatures in an oxygen-starved environment. This process breaks down complex organic materials into three distinct product types: a solid (biochar), a liquid (bio-oil), and a gas.
The Key Gaseous Components
The gas produced is a collection of non-condensable gases, meaning they remain in a gaseous state after the condensable liquids (like bio-oil and tar) have been separated out.
The primary components you will find in this mixture are:
- Hydrogen (H2): A clean-burning, high-energy fuel.
- Carbon Monoxide (CO): A combustible gas that releases energy when burned.
- Methane (CH4): The main component of natural gas, it has significant fuel value.
- Light Hydrocarbons (CnHm): Other simple organic gas molecules that contribute to the fuel value.
- Carbon Dioxide (CO2): A non-combustible byproduct.
Factors That Dictate the Final Output
You cannot consider the gas output in isolation. It is part of a system where adjusting one variable changes the yield of all three products (solid, liquid, and gas).
The Role of Feedstock
The material you start with fundamentally changes the output. The chemical makeup of the feedstock determines the building blocks available for the final products.
For example, the pyrolysis of biomass like wood will produce a balanced mix of char, oil, and syngas. In contrast, the pyrolysis of methane is a specialized process designed specifically to produce solid carbon and high-purity gaseous hydrogen.
The Impact of Process Temperature
Temperature is the most significant control lever for determining the product yields. There is a direct relationship between heat and the state of the final products.
Lower temperatures, typically around 400–500 °C, favor the production of the solid product, biochar. The complex molecules don't have enough energy to break down completely.
As the temperature increases above 700 °C, the feedstock molecules break down more thoroughly into simpler, lighter molecules, which favors the production of liquid bio-oil and gaseous syngas.
Understanding the Practical Applications
The gas produced during pyrolysis is rarely a waste product. It is an integral part of the process and a valuable output with several key uses.
Powering the Pyrolysis Process
The most common application for pyrolysis gas is to use it as a fuel source to generate the heat required for the pyrolysis reactor itself. This creates a self-sustaining energy loop, significantly reducing the external energy required to run the plant.
Generating Heat and Electricity
If there is a surplus of gas beyond what is needed to sustain the reaction, it can be used for other purposes. It can be burned in a boiler to generate heat for industrial processes or used to power an engine or turbine to produce electricity.
Making the Right Choice for Your Goal
The "best" pyrolysis setup depends entirely on the desired end product. You must tune the process to match your primary objective.
- If your primary focus is carbon sequestration or soil amendment: Operate at lower temperatures (400-500°C) with a biomass feedstock to maximize the yield of solid biochar.
- If your primary focus is producing liquid fuels: Operate at higher temperatures (above 700°C) to favor the cracking of materials into the condensable liquids that form bio-oil.
- If your primary focus is generating clean hydrogen fuel: Utilize a specialized process like methane pyrolysis, which is specifically designed to split methane into hydrogen gas and solid carbon.
Ultimately, pyrolysis is a versatile thermochemical tool for converting a wide range of materials into more valuable energy and chemical products.
Summary Table:
| Gas Component | Description | Common Feedstock Examples |
|---|---|---|
| Hydrogen (H₂) | Clean-burning, high-energy fuel | Methane, Biomass |
| Methane (CH₄) | Main component of natural gas | Biomass, Plastics |
| Carbon Monoxide (CO) | Combustible gas for energy | Biomass, Waste Materials |
| Carbon Dioxide (CO₂) | Non-combustible byproduct | All organic materials |
| Light Hydrocarbons (CₙHₘ) | Simple organic gas molecules | Plastics, Rubber |
Ready to Harness the Power of Pyrolysis Gas?
Whether you're developing a pyrolysis system for waste conversion, biofuel production, or hydrogen generation, having the right laboratory equipment is crucial for process optimization and analysis.
KINTEK specializes in supplying high-quality lab equipment and consumables to support your pyrolysis R&D and quality control needs. From gas analyzers and temperature control systems to reactor components, we provide the reliable tools you need to accurately monitor and control your pyrolysis process.
Let us help you achieve your sustainability and energy goals. Our experts can recommend the right equipment for analyzing syngas composition, optimizing yields, and scaling your process.
Contact us today to discuss your specific pyrolysis application and equipment requirements!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the different types of pyrolysis machines? Choose the Right System for Your Output



















