In chemical vapor deposition (CVD), the gases used are known as precursors, and they are specifically chosen volatile compounds that contain the elements intended for deposition. These precursors are transported into a reaction chamber where they decompose or react on a heated substrate surface, leaving behind a thin film of the desired material. The exact gas depends entirely on the film you intend to create, ranging from silane for silicon to complex metalorganics for advanced electronic components.
The core principle is that the choice of gas is not arbitrary; it is a precise chemical recipe. The precursor gas acts as the fundamental building block, and its chemical properties directly dictate the composition of the final deposited film and the conditions required for the process.

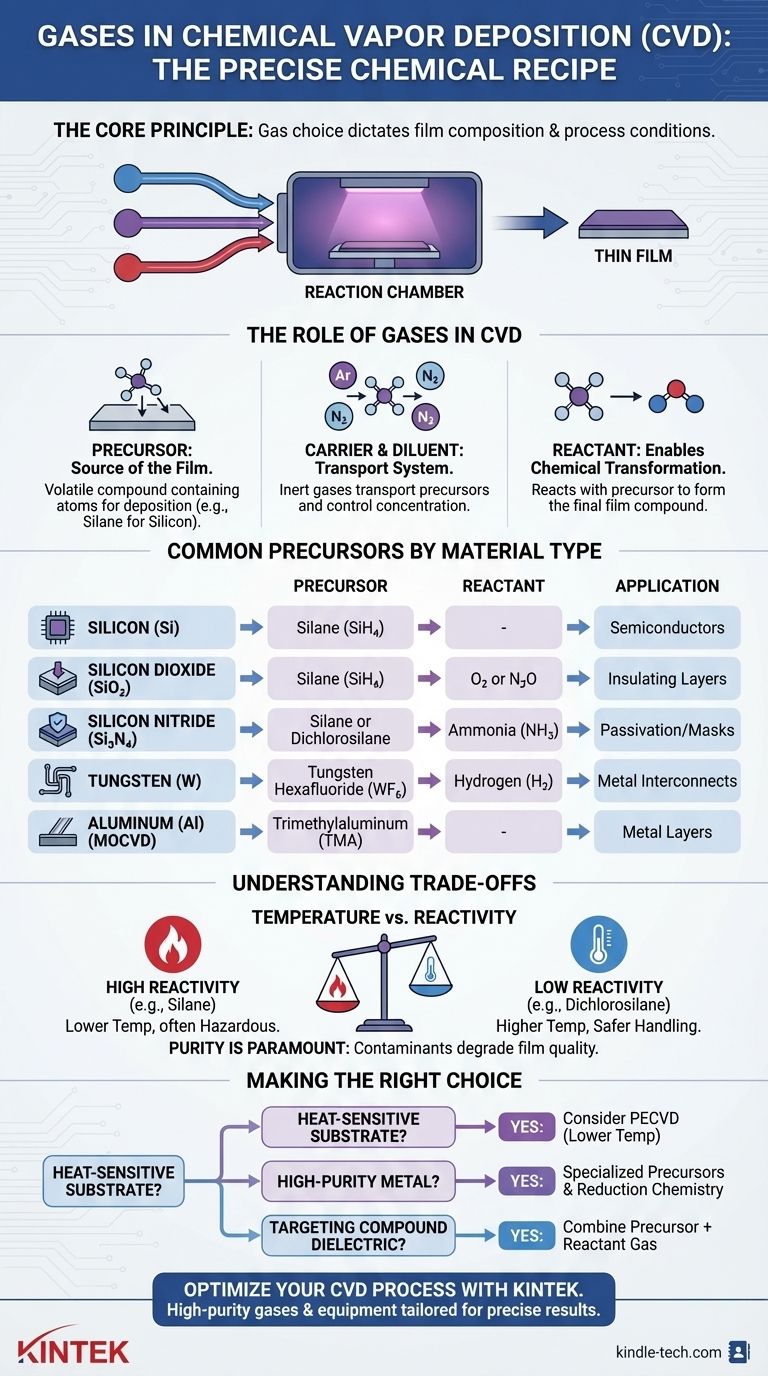
The Role of Gases in the CVD Process
Gases are the lifeblood of any CVD process. They aren't just one component; they serve distinct functions within the reaction chamber to enable controlled film growth. Understanding these roles is key to understanding CVD itself.
The Precursor: The Source of the Film
The most critical gas is the precursor. This is a volatile compound that contains the atoms you want to deposit.
It is designed to be stable at room temperature for transport but reactive enough to decompose or react at the substrate under specific conditions (heat, plasma, or light). For example, to deposit a silicon film, a silicon-containing precursor is required.
Carrier and Diluent Gases: The Transport System
Precursors are often highly concentrated or reactive. To control the process, they are mixed with other gases.
Carrier gases, such as argon (Ar), helium (He), nitrogen (N₂), or hydrogen (H₂), are inert. Their job is to transport the precursor molecules to the substrate surface without participating in the chemical reaction.
Diluent gases serve a similar transport function but also help control the concentration of reactants, which directly influences the deposition rate and film uniformity.
Reactant Gases: Enabling Chemical Transformation
In many CVD processes, the precursor doesn't just decompose; it reacts with another gas to form the final film.
For instance, to create silicon nitride (Si₃N₄), a silicon precursor like silane (SiH₄) is introduced along with a nitrogen-source reactant gas like ammonia (NH₃). The chemical reaction between these two gases at the surface forms the desired compound film.
Common Precursor Gases by Material Type
The specific gas used is determined by the target material. Below are common examples that illustrate this direct relationship.
For Silicon Films (Si)
Silicon is the foundation of the semiconductor industry. The most common precursor is silane (SiH₄). At elevated temperatures, it decomposes, leaving a solid silicon film and releasing hydrogen gas. Other silicon precursors like dichlorosilane (SiH₂Cl₂) are used for different film properties or deposition conditions.
For Dielectric and Insulating Films
Dielectrics are essential for insulating components in microelectronics.
- Silicon Dioxide (SiO₂): Often deposited using silane (SiH₄) with an oxygen source like oxygen (O₂) or nitrous oxide (N₂O).
- Silicon Nitride (Si₃N₄): Typically deposited using silane (SiH₄) or dichlorosilane (SiH₂Cl₂) in combination with ammonia (NH₃).
For Metal and Conductive Films
CVD is also used to deposit conductive metal layers.
- Tungsten (W): The most common precursor is tungsten hexafluoride (WF₆), which is reduced by hydrogen (H₂) to deposit a pure tungsten film.
- Aluminum (Al): Often deposited using metalorganic precursors, such as trimethylaluminum (TMA). This class of precursors is known as metalorganic chemical vapor deposition (MOCVD).
Understanding the Trade-offs
The choice of precursor is a critical engineering decision involving significant trade-offs. There is no single "best" gas; the right choice depends on the specific application and process limitations.
Temperature vs. Reactivity
Highly reactive precursors like silane can deposit films at lower temperatures but are often pyrophoric (ignite spontaneously in air) and hazardous to handle. Less reactive precursors, like dichlorosilane, are safer but require higher process temperatures, which can damage other components on the substrate.
Purity and Film Quality
The purity of the precursor gas is paramount, as any contaminants can be incorporated into the growing film, degrading its performance. Some precursors may also leave behind undesirable elements (like carbon or chlorine), which must be managed through careful process tuning.
The Role of Process Type
The type of CVD process influences precursor choice. Plasma-Enhanced CVD (PECVD) uses plasma to help break down the precursor gases. This allows deposition to occur at much lower temperatures than traditional Thermal CVD, enabling the use of precursors that would be unsuitable for high-temperature processes.
Making the Right Choice for Your Goal
Selecting the correct gases is about matching the chemical precursors and reactants to the desired material outcome and process constraints.
- If your primary focus is depositing elemental silicon: Your starting point is almost always silane (SiH₄), with process temperature being the main variable.
- If your primary focus is creating a compound dielectric like silicon nitride: You must use a combination of a silicon precursor (like SiH₄) and a nitrogen reactant (like NH₃).
- If your primary focus is working with heat-sensitive substrates: You should investigate Plasma-Enhanced CVD (PECVD) processes, as they allow for high-quality films at significantly lower temperatures.
- If your primary focus is depositing high-purity metals: You will need to use specialized precursors like tungsten hexafluoride (WF₆) and understand the reduction chemistry involved.
Ultimately, mastering CVD requires you to think like a chemist, selecting the right gaseous ingredients to build your desired material one atomic layer at a time.
Summary Table:
| Material Type | Common Precursor Gases | Reactant Gases | Common Applications |
|---|---|---|---|
| Silicon (Si) | Silane (SiH₄), Dichlorosilane (SiH₂Cl₂) | - | Semiconductors, Microelectronics |
| Silicon Dioxide (SiO₂) | Silane (SiH₄) | Oxygen (O₂), Nitrous Oxide (N₂O) | Insulating Layers |
| Silicon Nitride (Si₃N₄) | Silane (SiH₄), Dichlorosilane (SiH₂Cl₂) | Ammonia (NH₃) | Hard Masks, Passivation |
| Tungsten (W) | Tungsten Hexafluoride (WF₆) | Hydrogen (H₂) | Metal Interconnects |
| Aluminum (Al) | Trimethylaluminum (TMA) | - | Metal Layers (MOCVD) |
Optimize Your CVD Process with KINTEK
Choosing the right precursor gases is critical for achieving high-quality, uniform thin films in your laboratory. Whether you are depositing silicon for semiconductors, dielectrics for insulation, or metals for interconnects, the correct gas selection and process parameters are key to your success.
KINTEK specializes in providing high-purity lab gases, CVD equipment, and consumables tailored to your specific research and production needs. Our expertise ensures you have the reliable materials and support necessary to achieve precise and repeatable results.
Ready to enhance your deposition process? Contact our experts today to discuss your CVD requirements and discover how KINTEK can support your laboratory's success.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- Why are carbon nanotubes important in industry? Unlocking Next-Generation Material Performance
- What is the floating catalyst method? A Guide to High-Yield CNT Production
- How does chirality affect carbon nanotubes? It Determines If They Are Metal or Semiconductor
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- How high of temperature do carbon nanotubes in air have the ability to sustain? Understanding the Oxidation Limit



















