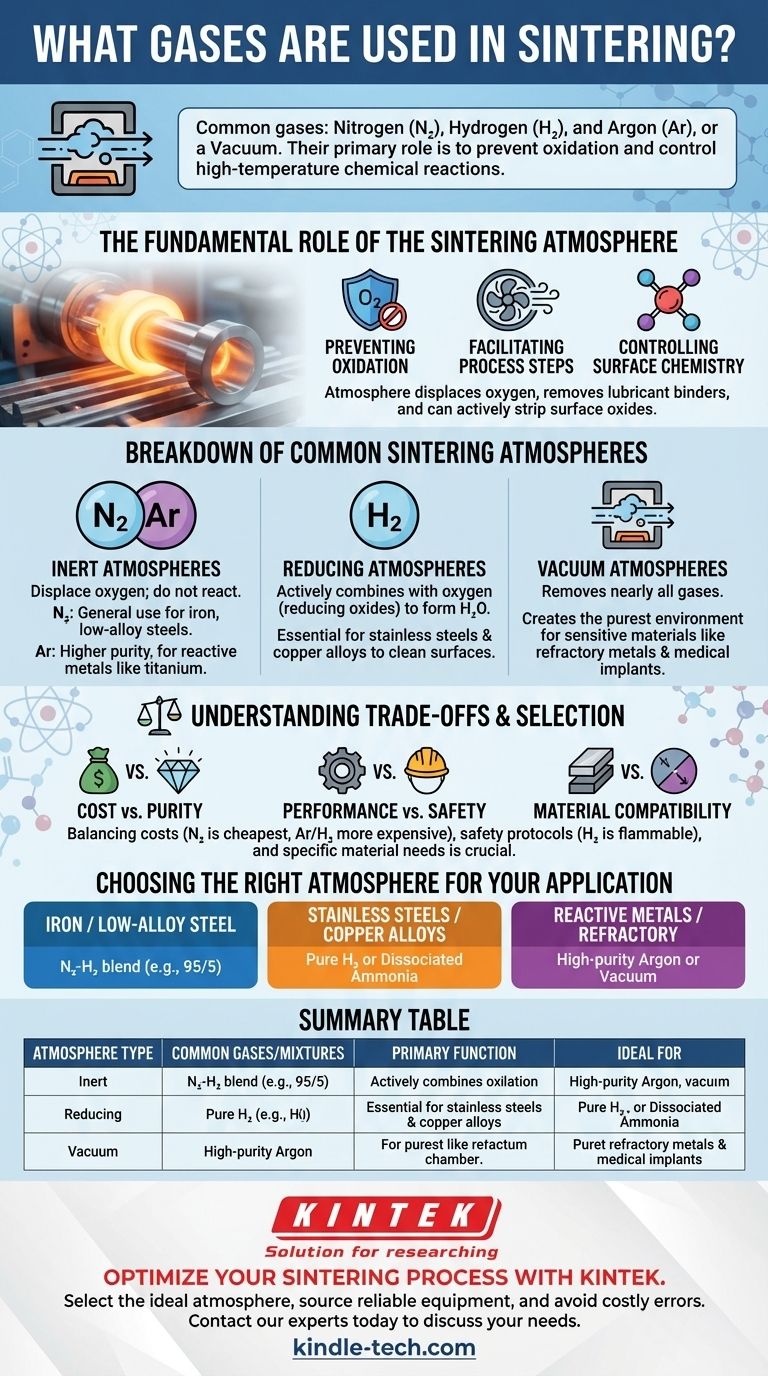
In short, the most common gases used in sintering are Nitrogen (N₂), Hydrogen (H₂), and Argon (Ar), often used alone, in mixtures, or as a component of a dissociated ammonia atmosphere. A vacuum is also frequently used as an "atmosphere" to remove reactive gases entirely. The choice depends entirely on the material being processed and the desired chemical outcome, as the atmosphere's primary roles are to prevent oxidation and control chemical reactions at high temperatures.
The atmosphere inside a sintering furnace is not a passive environment; it is an active ingredient in the process. Its fundamental purpose is to control the chemical conditions at elevated temperatures, preventing destructive oxidation and ensuring the metallurgical integrity of the final part.

The Fundamental Role of the Sintering Atmosphere
Sintering involves heating compacted powders to temperatures just below their melting point. The references describe this as a process of particle diffusion, neck formation, and densification to form a solid mass. At these high temperatures, however, most metals become extremely reactive.
Preventing Oxidation and Contamination
The primary job of a sintering atmosphere is to displace the oxygen found in ambient air. If present, oxygen would rapidly form oxides on the surface of the metal particles, preventing them from properly bonding and severely degrading the mechanical properties of the final component.
The controlled atmosphere creates an environment that is either chemically non-reactive (inert) or actively beneficial (reducing).
Facilitating Process Steps
As raw materials are heated, lubricants and binders used during the powder compaction stage must be burned off and removed. The flowing gas of the sintering atmosphere acts as a carrier, sweeping these vapors out of the furnace to prevent them from contaminating the parts.
Controlling Surface Chemistry
Beyond just preventing unwanted reactions, certain atmospheres can be used to drive desirable chemical changes. For example, a reducing atmosphere can actively strip away pre-existing surface oxides that may have formed on the powder particles before the sintering process even began.
A Breakdown of Common Sintering Atmospheres
The selection of a specific gas or gas mixture is a critical engineering decision based on the material being sintered, the required final properties, and cost.
Inert Atmospheres: Nitrogen and Argon
Nitrogen (N₂) and Argon (Ar) are inert gases, meaning they do not readily react with other elements. Their main function is to displace oxygen.
- Nitrogen is the most common and cost-effective choice for general-purpose sintering of iron and low-alloy steels.
- Argon is more expensive but is also denser and more purely inert than nitrogen. It is reserved for highly reactive materials like titanium, certain stainless steels, or superalloys that could form undesirable nitrides if processed in a nitrogen atmosphere.
Reducing Atmospheres: Hydrogen
Hydrogen (H₂) is a reactive gas, but its reactivity is highly beneficial in sintering. It actively seeks out and combines with oxygen (reducing it) to form water vapor (H₂O), which is then carried out of the furnace.
This makes hydrogen exceptionally effective at cleaning surface oxides from metal particles, promoting a stronger metallic bond. It is essential for materials with easily oxidized elements, like the chromium in stainless steel. Hydrogen is often mixed with nitrogen in various ratios (e.g., 90% N₂ / 10% H₂) to balance cost and performance.
Vacuum Atmospheres
A vacuum is the ultimate "inert" atmosphere, created by physically removing nearly all gas molecules from the furnace chamber. This provides the purest possible environment, free from potential contamination.
Vacuum sintering is used for the most sensitive and reactive materials, such as refractory metals, certain tool steels, and medical implants, where even trace amounts of gas could compromise performance.
Understanding the Trade-offs
Choosing an atmosphere involves balancing material requirements, operational costs, and safety protocols. There is no single "best" gas for all applications.
Cost vs. Purity
Nitrogen is relatively inexpensive, while pure hydrogen and especially argon are significantly more costly. Running a vacuum furnace also involves higher capital and operational expenses compared to an atmospheric furnace. The cost must be justified by the material's requirements.
Performance vs. Safety
Hydrogen is a superior reducing agent but is highly flammable and requires stringent safety systems. It can also cause hydrogen embrittlement in certain high-carbon or high-hardness steels, which limits its use in some applications. Inert gases are safer but lack hydrogen's active cleaning properties.
Material Compatibility is Non-Negotiable
The wrong atmosphere can ruin a part. Using a nitrogen-based atmosphere to sinter a titanium part will create brittle titanium nitrides. Sintering stainless steel in an atmosphere without sufficient reducing potential (like pure, dry hydrogen or a vacuum) will fail to remove chromium oxides, resulting in poor sintering.
Choosing the Right Atmosphere for Your Application
Your choice must be dictated by the chemistry of the material you are processing.
- If your primary focus is cost-effective sintering of iron or low-alloy steel: A nitrogen-hydrogen blend (e.g., 95/5) is the industry standard, offering good performance at a manageable cost.
- If your primary focus is sintering stainless steels, tool steels, or copper alloys: A pure, dry hydrogen atmosphere or a dissociated ammonia atmosphere is required to effectively reduce surface oxides.
- If your primary focus is sintering highly reactive metals like titanium or refractory metals: A high-purity argon atmosphere or a high-quality vacuum is non-negotiable to prevent any contamination.
Ultimately, selecting the correct sintering atmosphere is a foundational decision that directly controls the metallurgical quality and performance of the final component.
Summary Table:
| Atmosphere Type | Common Gases/Mixtures | Primary Function | Ideal For |
|---|---|---|---|
| Inert | Nitrogen (N₂), Argon (Ar) | Displaces oxygen to prevent oxidation | Iron, low-alloy steels (N₂); Titanium, reactive alloys (Ar) |
| Reducing | Hydrogen (H₂), N₂/H₂ Blends | Actively removes surface oxides | Stainless steels, copper alloys, tool steels |
| Vacuum | N/A (removal of gases) | Provides ultra-pure, contamination-free environment | Refractory metals, medical implants, sensitive alloys |
Optimize Your Sintering Process with KINTEK
Choosing the correct sintering atmosphere is critical to achieving the desired mechanical properties and metallurgical integrity in your final components. The wrong choice can lead to oxidation, contamination, or poor bonding, ruining an entire production batch.
KINTEK is your partner in precision sintering. We specialize in providing the high-quality lab equipment and expert consumables needed to control your sintering environment perfectly. Whether your process requires cost-effective nitrogen blends, high-purity hydrogen, or inert argon atmospheres, we have the solutions and support to ensure your success.
Let us help you:
- Select the ideal atmosphere for your specific material, from stainless steel to titanium.
- Source reliable gases and equipment to maintain a consistent, high-purity environment.
- Avoid costly errors and achieve superior part density and strength.
Don't let atmosphere selection compromise your results. Contact our experts today to discuss your sintering needs and discover how KINTEK can enhance your laboratory's capabilities.
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Graphite Vacuum Furnace Negative Material Graphitization Furnace
People Also Ask
- How is the greatest joint strength obtained in brazing? Master the 3 Keys to Superior Metallurgical Bonds
- How is a high-vacuum or atmosphere sintering furnace utilized for nanocrystalline stainless steel thermal stability?
- Is brazing environmentally friendly? A Guide to Sustainable, Low-Impact Joining
- What is the cost of a plasma pyrolysis machine? Key Factors That Determine Your Investment
- What is the difference between calcination and sintering furnace? A Guide to Thermal Processing Goals
- What is the function of a vacuum arc melting furnace? Prepare High-Purity Alx(CrFeNi)1-x High-Entropy Alloys
- What equipment is used for heat treatment of steel? Choose the Right Furnace for Your Process
- What are the advantages of torch brazing? Discover the Superior Control of Modern Brazing



















