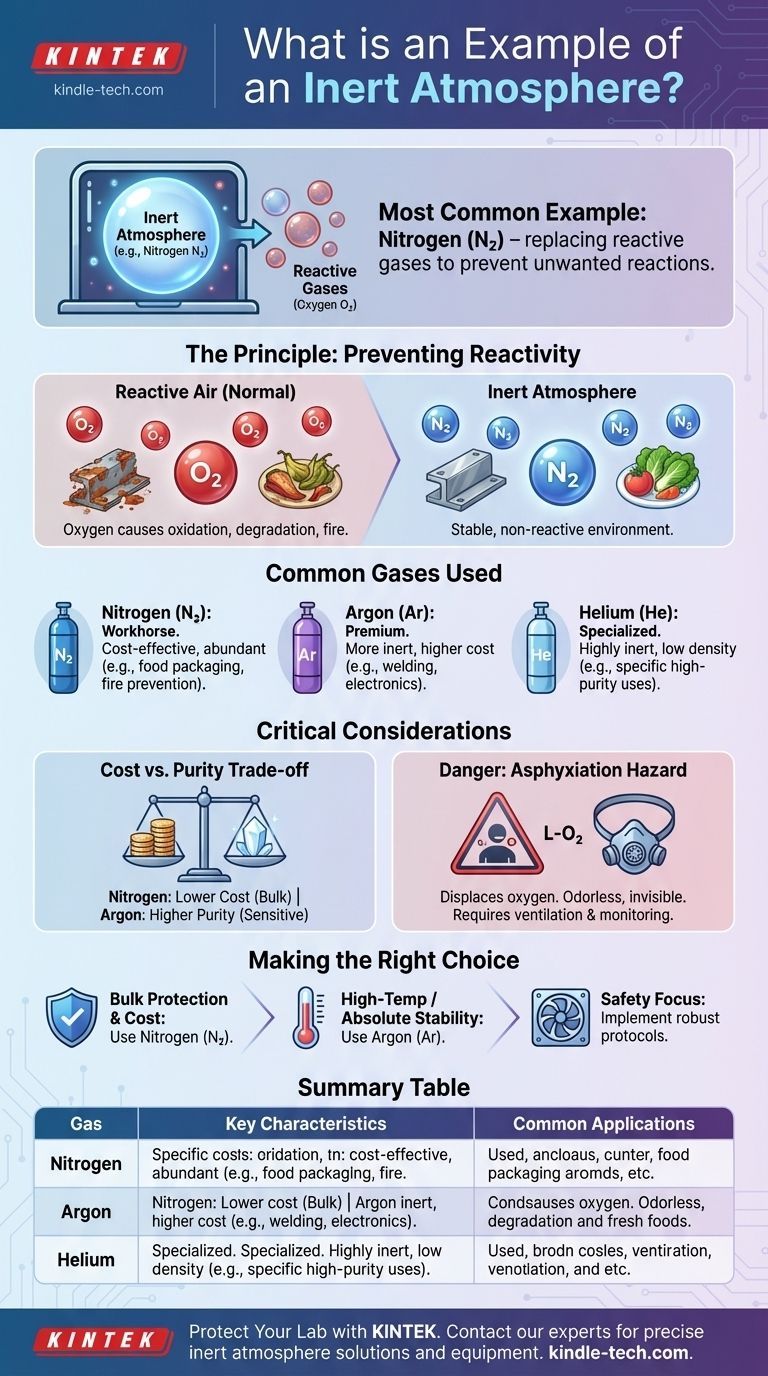
The most common example of an inert atmosphere is one created using pure nitrogen gas (N₂). This is because nitrogen is largely non-reactive under most conditions and makes up about 78% of the air we breathe, making it an abundant and cost-effective choice for preventing unwanted chemical changes.
An inert atmosphere is less about a specific gas and more about a strategy: replacing reactive gases like oxygen with a non-reactive gas to prevent unwanted chemical reactions, such as oxidation or degradation, in a sensitive environment.

The Principle of Inerting: Preventing Unwanted Reactions
To understand inert atmospheres, you must first understand the problem they solve. The standard air around us is a reactive mixture, primarily of nitrogen, oxygen, and water vapor.
What "Inert" Really Means
The term "inert" refers to a substance that is chemically non-reactive. In the context of gases, this means the gas has a very stable electron configuration and does not easily form chemical bonds with other materials.
These gases have low oxidation potentials, meaning they do not readily cause or participate in oxidation—the process responsible for rust, food spoilage, and combustion.
The Problem: A Reactive Atmosphere
The primary culprit in a normal atmosphere is oxygen. Oxygen is highly reactive and will aggressively combine with other substances.
This reactivity is the source of many problems in industrial and scientific processes, including metal corrosion, degradation of sensitive chemicals, and the risk of fire or explosion with flammable materials.
Common Gases Used for Inerting
While nitrogen is the most common, other gases are used based on specific needs.
- Nitrogen (N₂): The workhorse of inerting. It is cost-effective and suitable for a vast range of applications, from food packaging to electronics manufacturing.
- Argon (Ar): More inert than nitrogen, but also more expensive. It is used in high-temperature or highly sensitive applications like welding reactive metals (e.g., titanium) and growing silicon crystals.
- Helium (He): Also highly inert, but it is a very small, low-density molecule that can be difficult to contain. Its use is more specialized.
Understanding the Trade-offs
Choosing to use an inert atmosphere involves practical considerations, and not all gases are suitable for the job.
Why Some Gases Are Unsuitable
A gas like chlorine is an excellent example of a non-inert gas. As a halogen, it is extremely reactive and will readily attack other materials.
Furthermore, its toxicity makes it fundamentally unsuitable for creating a safe, stable environment, highlighting that an inerting gas must be both chemically stable and safe to handle.
Cost vs. Purity of Inertness
The primary trade-off is often between cost and the required level of inertness.
Nitrogen is produced by separating it from air, making it relatively inexpensive for bulk applications. Argon, which makes up less than 1% of the atmosphere, is more costly to isolate and is therefore reserved for processes where nitrogen is not sufficiently non-reactive.
The Critical Danger: Asphyxiation
A crucial point of safety is that an inert atmosphere, by definition, displaces oxygen. In any enclosed or poorly ventilated space, a buildup of any inert gas creates an asphyxiation hazard.
These atmospheres cannot support life and are dangerous because they are often colorless and odorless, providing no sensory warning of the oxygen-deficient environment.
Making the Right Choice for Your Goal
Selecting the correct inert gas depends entirely on the technical requirements, budget, and safety protocols of your project.
- If your primary focus is bulk protection and cost-efficiency: Nitrogen is the definitive choice for applications like food packaging, blanketing chemical storage tanks, and preventing fires.
- If your primary focus is high-temperature stability or absolute non-reactivity: Argon is necessary for sensitive processes like TIG/MIG welding of special alloys or manufacturing advanced electronics.
- If your primary focus is personnel safety: Any inert gas strategy must be accompanied by robust ventilation, air monitoring for oxygen levels, and strict access protocols.
By understanding the principle of chemical reactivity, you can confidently select the right inerting strategy to protect your materials and processes.
Summary Table:
| Gas | Key Characteristics | Common Applications |
|---|---|---|
| Nitrogen (N₂) | Cost-effective, abundant, suitable for most uses | Food packaging, chemical storage, fire prevention |
| Argon (Ar) | Highly inert, excellent for high temperatures | Welding reactive metals, electronics manufacturing |
| Helium (He) | Highly inert, low density, can be difficult to contain | Specialized applications requiring high purity |
Need to protect your materials from oxidation, spoilage, or fire?
Choosing the right inert atmosphere is critical for your lab's success and safety. KINTEK specializes in providing the precise lab equipment and consumables you need to create and maintain controlled environments.
Our experts can help you select the optimal solution for your specific application, whether you're working with sensitive chemicals, conducting high-temperature processes, or ensuring product longevity.
Contact our team today to discuss your inert atmosphere requirements and discover how KINTEK can enhance your laboratory's efficiency and safety.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why Use an Atmosphere Protection Furnace with Argon for FM Steel? Ensure Integrity and Prevent Oxidation
- What is the atmosphere of a heat treatment furnace? Mastering Surface Chemistry for Superior Materials
- What does inert atmosphere mean in science? Control Chemical Reactions and Ensure Process Safety
- How an Atmosphere Tube Furnace Prepares Oxygen-Deficient RPPO via Reduction: Achieve Superior Ionic Conductivity
- What is the use of annealing process in metal industry? Relieve Stress and Increase Ductility for Manufacturing
- What are the important applications of inert gases? Essential Uses from Welding to Food Preservation
- How does the chemical reduction of silica during hydrogen sintering affect the furnace's refractory materials? Ensure Longevity with the Right Lining
- What is the function of a high-temperature atmosphere tube furnace in P-NCS synthesis? Expert Insights



















