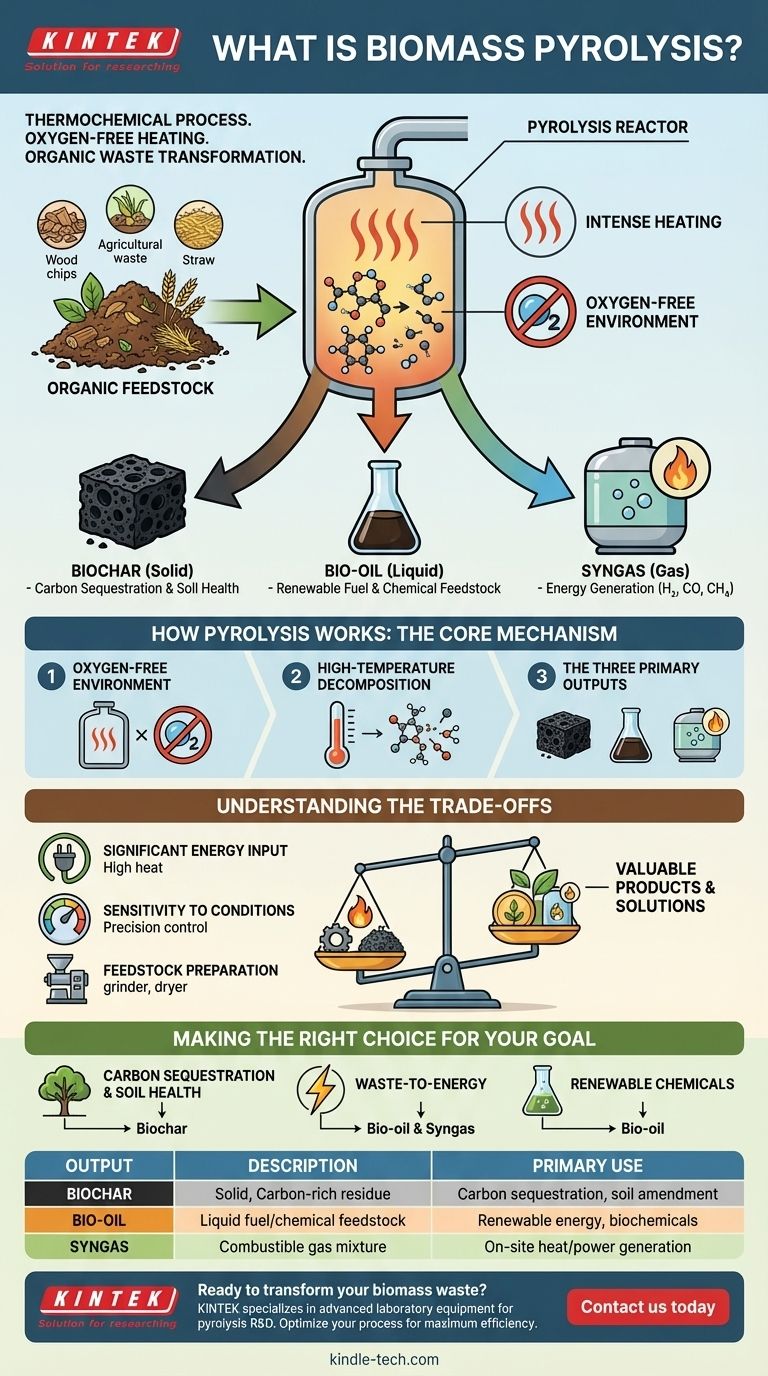
At its core, biomass pyrolysis is a thermochemical process that rapidly heats organic materials like wood or agricultural waste in an oxygen-free environment. This intense heating, without actual burning, breaks down the complex structure of the biomass. This decomposition transforms it into more stable and valuable products.
The fundamental goal of biomass pyrolysis is not to destroy waste, but to chemically upgrade it. By carefully controlling heat in the absence of oxygen, the process locks carbon into a solid form (biochar) while also creating liquid (bio-oil) and gaseous (syngas) fuels.

How Pyrolysis Works: The Core Mechanism
Pyrolysis is a controlled decomposition process, distinct from simple burning or incineration. The entire system is engineered to capture and separate the valuable outputs created when biomass is broken down by heat.
The Oxygen-Free Environment
The most critical condition for pyrolysis is the absence of oxygen. The biomass is fed into a fully sealed reactor, which prevents combustion.
Instead of burning and releasing its energy as light and heat, the biomass undergoes a chemical transformation. This preserves a significant portion of the original carbon.
High-Temperature Decomposition
Inside the reactor, the material is heated to high temperatures. This intense thermal energy breaks the complex chemical bonds within the biomass.
The process effectively "cracks" large organic molecules into smaller, less complex ones, separating the original material into solid, liquid, and gas components.
The Three Primary Outputs
The specific yields of each product can be adjusted by changing process conditions, but pyrolysis always produces three distinct outputs.
- Biochar (Solid): This is the solid, charcoal-like residue. It contains most of the original carbon and any non-volatile components from the biomass.
- Bio-oil (Liquid): This liquid, also known as pyrolysis oil or wood acid, is a complex mixture of water and organic compounds. It is formed when the hot vapors from the reaction are rapidly cooled and condensed.
- Syngas (Gas): This is a mix of non-condensable gases, including hydrogen, carbon monoxide, and methane. This gas mixture has heating value and can be combusted for energy.
Understanding the Trade-offs
While powerful, pyrolysis is not a perfect solution. Understanding its operational requirements and limitations is key to evaluating its practical application.
Significant Energy Input
Pyrolysis is an energy-intensive process. It requires a substantial amount of external energy to get the reactor to the necessary high temperatures and maintain them.
Often, the syngas produced is combusted on-site to provide this heat, making the system more self-sufficient but reducing the amount of energy available for export.
Sensitivity to Conditions
The final output is highly dependent on the exact process conditions. Factors like temperature, heating rate, and the type of biomass feedstock drastically alter the ratio of biochar, bio-oil, and syngas produced.
This requires sophisticated control systems to ensure the process is optimized for the desired outcome, whether that's maximizing biochar for carbon sequestration or bio-oil for fuel.
Feedstock Preparation
Raw biomass is often not suitable for direct use. It typically must be dried to a low moisture content and shredded or pelletized to a uniform size before being fed into the reactor.
These pre-processing steps add complexity, cost, and energy consumption to the overall operation.
Making the Right Choice for Your Goal
Pyrolysis is a versatile platform, not a single solution. The value it provides depends entirely on your end goal.
- If your primary focus is carbon sequestration and soil health: The process is exceptionally effective at producing biochar, a stable form of carbon that can improve soil quality and lock away atmospheric CO2 for centuries.
- If your primary focus is waste-to-energy: Pyrolysis offers a method to convert low-value organic waste into bio-oil and syngas, which can be used to generate electricity or heat.
- If your primary focus is producing renewable chemicals: The condensed bio-oil can serve as a feedstock for refineries, where it can be upgraded into advanced biofuels or valuable biochemicals.
Ultimately, biomass pyrolysis provides a powerful technological pathway for transforming organic waste from a liability into a valuable resource.
Summary Table:
| Output | Description | Primary Use |
|---|---|---|
| Biochar | Solid, carbon-rich residue | Carbon sequestration, soil amendment |
| Bio-Oil | Liquid fuel/chemical feedstock | Renewable energy, biochemicals |
| Syngas | Combustible gas mixture (H₂, CO, CH₄) | On-site heat/power generation |
Ready to transform your biomass waste into valuable resources?
KINTEK specializes in advanced laboratory equipment for pyrolysis research and development. Whether you're focused on carbon sequestration, renewable energy, or biochemical production, our solutions help you optimize your pyrolysis process for maximum efficiency and yield.
Contact us today to discuss how our lab equipment can support your biomass conversion goals!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What criteria determine whether to use a vacuum tube furnace or a vacuum chamber furnace? Scale and Temperature Are Key
- What environment do high-temperature tube furnaces provide for N10276 alloy research? Precision Simulation for Alloys
- What are the main types of biomass conversion processes? Unlock the Best Pathway for Your Energy Needs
- What is the main problem with vacuum tubes? Inefficiency, Heat, and Fragility Explained
- Why must a tube furnace with vacuum or inert protection be used for CTMSS? Key to Hydrothermal Stability
- What is the function of a high-temperature tube furnace? Simulate Nuclear Environments for Coating Tests
- Why must metal membrane coatings undergo annealing in a tube furnace? Enhance Adhesion and Structural Integrity
- What are the advantages of using U-shaped quartz reactors? Boost Accuracy in CO2 Hydrogenation & Kinetic Studies



















