At its core, the working mechanism of Chemical Vapor Deposition (CVD) is a process where a solid material is built atom by atom on a surface. Precursor gases containing the required chemical elements are introduced into a reaction chamber, where they decompose and react on a heated object, known as a substrate, to form a high-purity, solid thin film.
The essential principle of CVD is not merely coating a surface, but rather conducting a controlled chemical reaction directly on that surface. It uses energy—typically heat or plasma—to break down specific gas molecules and reassemble their constituent atoms into a new, solid material.

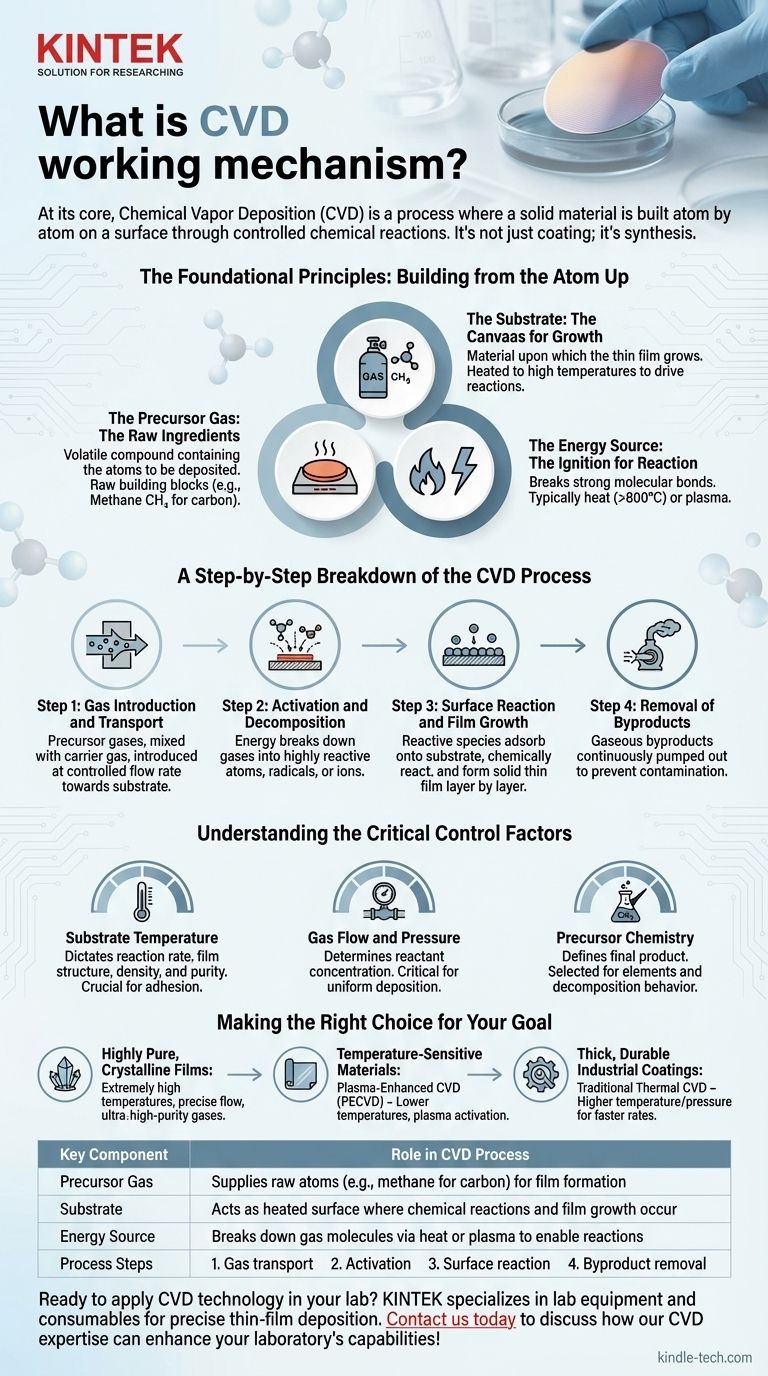
The Foundational Principles: Building from the Atom Up
To truly understand CVD, you must think of it as a form of chemical synthesis occurring in a highly controlled environment. It relies on three fundamental components working in concert.
The Precursor Gas: The Raw Ingredients
The precursor gas (or reactant gas) is a volatile compound that contains the atoms you wish to deposit. These gases are the raw building blocks for the final film.
For example, when creating synthetic diamonds, a carbon-rich gas like methane (CH₄) is used. This gas carries the necessary carbon atoms into the chamber.
The Substrate: The Canvas for Growth
The substrate is the material upon which the thin film is grown. It is not merely a passive holder; its surface provides the physical foundation for the film.
Crucially, the substrate is heated to a specific, high temperature. This heat provides the energy needed to drive the chemical reactions and often makes the substrate itself a catalyst for the deposition process.
The Energy Source: The Ignition for Reaction
A significant amount of energy is required to break the strong molecular bonds within the precursor gases. This is the "ignition" that initiates the entire process.
The most common energy source is heat, with the substrate often heated to temperatures of 800°C or higher. In other variations, plasma, lasers, or hot filaments are used to ionize the gas, breaking it down into more reactive components at lower overall temperatures.
A Step-by-Step Breakdown of the CVD Process
The CVD mechanism can be understood as a sequence of four distinct physical and chemical events.
Step 1: Gas Introduction and Transport
Precursor gases, often mixed with an inert carrier gas, are introduced into a sealed reaction chamber at a precisely controlled flow rate. These gases are transported toward the heated substrate.
Step 2: Activation and Decomposition
As the precursor gases approach or contact the hot substrate, the energy breaks them down. The molecules decompose into highly reactive atoms, radicals, or ions.
Step 3: Surface Reaction and Film Growth
These reactive species adsorb (adhere) onto the substrate's surface. A chemical reaction takes place directly on this surface, forming a stable, solid material.
This new material builds up systematically, often in crystalline layers, creating the desired thin film. For diamond growth, pure carbon atoms from the decomposed methane gas attach to a diamond "seed" crystal.
Step 4: Removal of Byproducts
The chemical reactions on the surface also create gaseous byproducts. These waste gases are continuously pumped out of the chamber to maintain the purity of the environment and prevent contamination of the growing film.
Understanding the Critical Control Factors
The final properties of a CVD film are not accidental; they are the direct result of meticulously controlling the process variables. Mismanaging these factors is the most common point of failure.
Substrate Temperature
Temperature is arguably the single most important parameter. It dictates the rate of the chemical reaction and influences the film's structure, density, and purity. An incorrect temperature can lead to poor adhesion or the formation of the wrong material entirely.
Gas Flow and Pressure
The flow rates of the precursor and carrier gases, along with the chamber pressure, determine the concentration of reactants available at the substrate surface. This control is critical for achieving a uniform deposition rate across the entire substrate.
Precursor Chemistry
The choice of precursor gas fundamentally defines the final product. The chemistry must be selected not only for the elements it contains but also for its decomposition behavior at the desired process temperature and pressure.
Making the Right Choice for Your Goal
Understanding the CVD mechanism allows you to tailor the process to your specific application.
- If your primary focus is creating highly pure, crystalline films (like semiconductor layers or synthetic diamonds): You must prioritize extremely high substrate temperatures, precise gas flow control, and ultra-high-purity precursor gases.
- If your primary focus is coating a temperature-sensitive material (like certain polymers): You should investigate Plasma-Enhanced CVD (PECVD), which uses an energy-efficient plasma to activate the gases, allowing for deposition at significantly lower temperatures.
- If your primary focus is achieving thick, durable industrial coatings: Traditional thermal CVD is an excellent choice, as its higher temperature and pressure conditions often facilitate faster growth rates for robust films.
By mastering these fundamental principles, you can transform simple gases into advanced, high-performance materials with remarkable precision.
Summary Table:
| Key Component | Role in CVD Process |
|---|---|
| Precursor Gas | Supplies raw atoms (e.g., methane for carbon) for film formation |
| Substrate | Acts as heated surface where chemical reactions and film growth occur |
| Energy Source | Breaks down gas molecules via heat or plasma to enable reactions |
| Process Steps | 1. Gas transport 2. Activation 3. Surface reaction 4. Byproduct removal |
Ready to apply CVD technology in your lab? KINTEK specializes in lab equipment and consumables for precise thin-film deposition. Whether you need high-purity precursor gases, temperature-controlled substrates, or energy-efficient plasma systems, we provide tailored solutions for semiconductor, coating, and materials research. Contact us today to discuss how our CVD expertise can enhance your laboratory's capabilities!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- How is ALD different from CVD? Choose Between Atomic Precision and High-Speed Deposition
- What is the core function of the CVD deposition furnace? Master Bulk ZnS Production with Precision Control
- Why is a high-precision CVD or tube furnace required for CNT/copper composites? Optimize In-Situ Growth Results
- How are thin film nanoparticles prepared? A Guide to PVD and CVD Deposition Methods
- What is the pressure of sputtering process? Mastering the Key to High-Quality Thin Films
- What is the process of chemical vapor infiltration? A Guide to Creating High-Performance CMCs
- How does pressure affect deposition? Mastering the Key to High-Quality Film Growth
- How does Thermal LCVD work? Mastering Precision Localized Deposition and Direct Micro-Fabrication



















