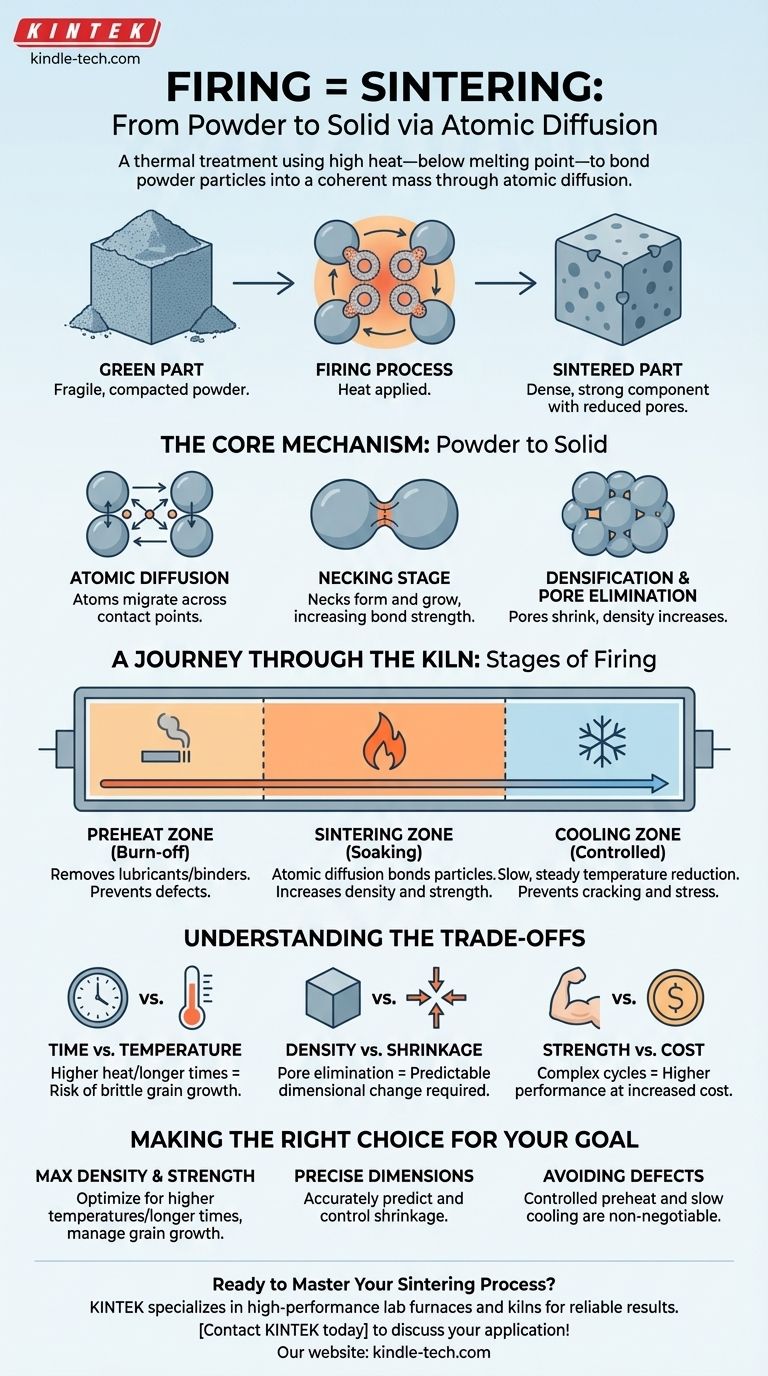
In materials science and manufacturing, firing and sintering refer to the exact same process. It is a thermal treatment that uses high heat—below the material's melting point—to bond a collection of individual powder particles into a solid, coherent mass. This transformation is driven by a phenomenon called atomic diffusion, where atoms move across the boundaries of the particles, effectively welding them together on a microscopic level.
The core takeaway is that firing, or sintering, is not about melting. It is a precise thermal process that uses atomic movement to transform a fragile, compacted powder part into a dense, strong, and engineered component by eliminating the spaces between the particles.

The Core Mechanism: From Powder to Solid
The journey begins with a "green part," which is a component formed by compacting fine powders. This green part is chalky and fragile, holding its shape but lacking any real structural strength. Firing is the critical step that provides that strength.
Atomic Diffusion at Work
Heat provides the energy for atoms at the surface of each powder particle to become mobile. These atoms migrate and diffuse across the contact points between adjacent particles, gradually filling the gaps and eliminating the boundaries that once separated them.
The Necking Stage
The process begins with the formation of "necks," which are small connection points where particles first start to fuse together. As the firing continues, these necks grow wider, increasing the bond strength between the particles.
Densification and Pore Elimination
As the necks grow and atoms continue to move, the empty spaces, or pores, between the original particles begin to shrink and close up. The ultimate goal is often to eliminate as many of these pores as possible, which significantly increases the density and strength of the final part.
A Journey Through the Kiln: The Stages of Firing
The sintering process is typically carried out in a high-temperature furnace or kiln, often with a precisely controlled atmosphere. The component moves through distinct temperature zones to ensure a successful transformation.
The Preheat Zone (Burn-off)
In the first zone, the part is heated slowly. The primary purpose here is to safely burn off any lubricants or organic binders that were used to hold the powder together in its initial "green" state. Rushing this step can cause defects.
The Sintering Zone (Soaking)
This is the hottest part of the kiln, where the actual sintering occurs. The component is held at a specific peak temperature for a set period, allowing atomic diffusion to bond the particles and densify the part.
The Cooling Zone (Controlled Cooling)
Finally, the part is cooled down in a controlled manner. A slow and steady cooling rate is crucial to prevent thermal shock, which can cause cracking and internal stresses, compromising the integrity of the newly formed component.
Understanding the Trade-offs
Sintering is a powerful process, but it involves a delicate balance of competing factors. Understanding these trade-offs is key to achieving the desired material properties.
Time vs. Temperature
Higher temperatures or longer firing times can accelerate densification. However, excessive heat can lead to undesirable grain growth, which can make the material brittle. The goal is to find the optimal combination that achieves density without compromising microstructure.
Density vs. Shrinkage
As the pores between particles are eliminated, the entire component shrinks. This shrinkage is significant and must be precisely calculated and accounted for during the initial design of the green part. Achieving high density always means managing dimensional change.
Strength vs. Cost
Longer, more complex firing cycles with highly controlled atmospheres produce superior parts but also increase manufacturing costs. The process must be engineered to meet the performance requirements without becoming economically unfeasible.
Making the Right Choice for Your Goal
The specifics of the firing cycle are tailored to the material and the desired outcome. Your primary objective will dictate your focus.
- If your primary focus is achieving maximum density and strength: You must optimize for higher temperatures and longer hold times in the sintering zone, while carefully managing the risk of grain growth.
- If your primary focus is maintaining precise dimensions: Your main challenge will be accurately predicting and controlling shrinkage by managing powder characteristics and the firing cycle.
- If your primary focus is avoiding defects: A controlled, gradual preheat and a slow cooling rate are non-negotiable to prevent cracks from binder burnout or thermal shock.
Mastering the firing process is fundamental to engineering advanced materials with tailored properties.
Summary Table:
| Process Stage | Key Action | Primary Outcome |
|---|---|---|
| Preheat (Burn-off) | Removes lubricants/binders | Prevents defects |
| Sintering (Soaking) | Atomic diffusion bonds particles | Increases density and strength |
| Cooling (Controlled) | Slow, steady temperature reduction | Prevents cracking and stress |
| Trade-off | Consideration | Impact |
| Time vs. Temperature | Higher heat or longer times | Risk of brittle grain growth |
| Density vs. Shrinkage | Pore elimination | Predictable dimensional change required |
| Strength vs. Cost | Complex cycles | Higher performance at increased cost |
Ready to Master Your Sintering Process?
Achieving the perfect balance of density, strength, and dimensional accuracy requires precise thermal control. KINTEK specializes in high-performance lab furnaces and kilns designed for reliable and repeatable sintering results.
We provide the equipment to help you:
- Optimize time and temperature profiles for your specific materials.
- Control atmosphere conditions to prevent oxidation and defects.
- Achieve consistent results batch after batch.
Let our experts help you select the right furnace for your R&D or production needs. Contact KINTEL today to discuss your application and get a personalized solution!
Visual Guide
Related Products
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What is the primary function of quartz tubes in halide electrolyte synthesis? Ensure Purity & Precise Stoichiometry
- What is the role of a tube furnace in the thermal treatment of argyrodite electrolytes? Master Ionic Conductivity
- How do you clean a quartz tube furnace? Prevent Contamination & Extend Tube Lifespan
- What role does a quartz tube furnace play in hBN synthesis? Optimize Your Chemical Vapor Deposition Results
- What happens when quartz is heated? A Guide to Its Critical Phase Transitions and Uses



















