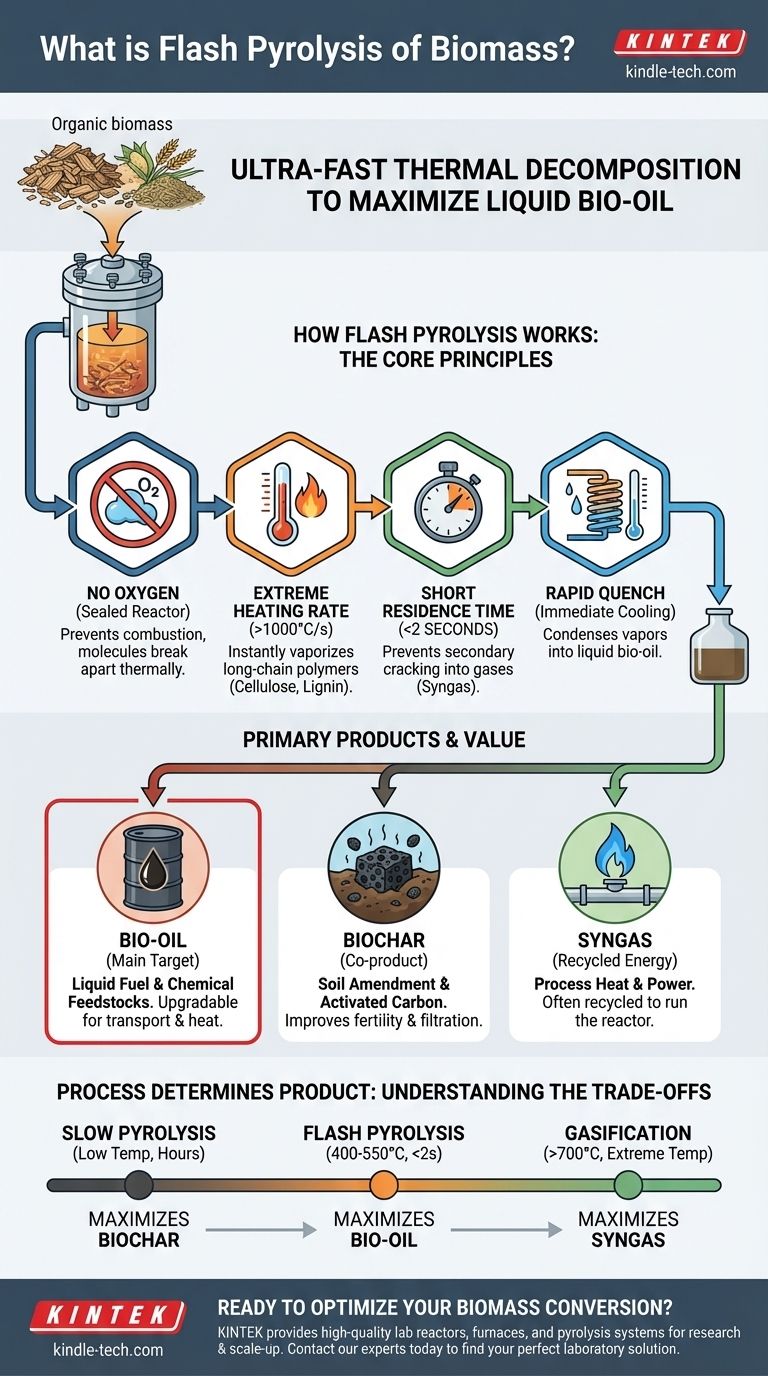
In essence, flash pyrolysis of biomass is an ultra-fast, high-temperature thermal decomposition process designed to maximize the production of liquid bio-oil. It works by heating organic material, such as agricultural waste or wood, to around 400-550°C in the complete absence of oxygen, with a vapor residence time of less than two seconds. This rapid heating and immediate cooling of the resulting vapors is the key to converting solid biomass primarily into a liquid fuel.
The critical takeaway is that process conditions dictate the final product. Flash pyrolysis manipulates speed and temperature to interrupt the natural decomposition process at the ideal moment, capturing valuable compounds as a liquid (bio-oil) before they can break down further into less valuable gases or consolidate into a solid (biochar).

How Flash Pyrolysis Works: The Core Principles
To understand flash pyrolysis, you must first understand it is thermal decomposition, not burning. The entire process is engineered around a few key variables to control the outcome.
The Absence of Oxygen
The process occurs inside a sealed reactor. By eliminating oxygen, we prevent combustion. Instead of burning and releasing energy as heat and light, the biomass molecules break apart under intense heat, rearranging into new, smaller molecules that form the products.
Extreme Heating Rate
This is the defining characteristic of flash pyrolysis. The biomass particles are heated incredibly quickly. This rapid energy transfer cracks the long-chain polymers like cellulose and lignin, vaporizing them almost instantly.
A slower heating rate would allow these molecules to rearrange into more stable, carbon-rich structures, forming more biochar. Speed is essential for maximizing liquid yield.
Short Residence Time
The resulting hot vapors and gases are removed from the reactor in less than two seconds. This is called a short residence time.
This step is crucial because it prevents "secondary cracking." If the hot vapors linger in the reactor, they will continue to break down into simpler, non-condensable gases (syngas), significantly reducing the final bio-oil yield. The vapors are rapidly cooled, or "quenched," to condense them into liquid bio-oil.
Controlled Temperature
The temperature range of 400-550°C is a carefully selected "sweet spot." It is hot enough to rapidly decompose the biomass but not so hot that it favors the production of gas over liquid.
The Primary Products and Their Value
The rapid, controlled nature of flash pyrolysis yields a specific mix of products, each with a distinct application. The process is almost always optimized to favor one product over the others.
Bio-oil (The Main Target)
Flash pyrolysis is engineered to produce the maximum possible amount of bio-oil, often making up the majority of the product mass. This dark, viscous liquid is a complex mixture of oxygenated organic compounds.
It can be upgraded into transportation fuels, used directly in some boilers and furnaces for heat and power, or serve as a source for valuable chemical feedstocks.
Biochar (A Key Co-product)
Biochar is the solid, carbon-rich residue left behind. While flash pyrolysis minimizes its production compared to other methods, it remains a valuable co-product.
Its primary applications are as a soil amendment to improve fertility and water retention or as a precursor for producing activated carbon used in filtration systems.
Syngas (Non-condensable Gases)
This is a mixture of gases like carbon monoxide, hydrogen, and methane. In most pyrolysis plants, this syngas is not wasted. It is often recycled and burned to provide the very heat needed to run the reactor, making the process more energy-efficient and self-sustaining.
Understanding the Trade-offs: Process Determines Product
The term "pyrolysis" covers a family of related processes. Choosing the right one depends entirely on your desired end product. The key difference between them is the heating rate and residence time.
The Goal Dictates the Process
You cannot maximize the yield of all three products simultaneously. The conditions that favor bio-oil production inherently limit the production of biochar, and vice versa.
Flash Pyrolysis for Bio-oil
As discussed, high heating rates and short residence times are used to maximize the liquid bio-oil yield. This is the pathway for producing biofuels and chemical feedstocks from biomass.
Slow Pyrolysis for Biochar
In contrast, slow pyrolysis uses a much lower heating rate over a period of hours. This gives the biomass time to slowly and thoroughly carbonize, maximizing the yield and quality of the solid biochar product.
Gasification for Syngas
To maximize the production of combustible gases, an even more extreme process called gasification is used. It involves higher temperatures (often >700°C) and sometimes the introduction of a small, controlled amount of oxygen or steam to promote the conversion of the biomass entirely into syngas.
Making the Right Choice for Your Goal
Selecting the correct thermal conversion technology is a direct function of your target output.
- If your primary focus is maximizing liquid biofuel or chemical feedstock: Flash pyrolysis is the definitive choice due to its rapid heating and short vapor residence time.
- If your primary focus is producing a stable soil amendment or solid fuel (biochar): A slower pyrolysis process with a longer residence time is the more effective and efficient pathway.
- If your primary focus is generating combustible gas (syngas) for power generation: Gasification, a related process operating at higher temperatures, is the most direct route.
Ultimately, mastering thermal conversion is about understanding how to manipulate heat and time to precisely control the deconstruction of biomass.
Summary Table:
| Process Characteristic | Flash Pyrolysis Setting | Purpose |
|---|---|---|
| Heating Rate | Very High (>1000°C/s) | Instantly vaporizes biomass to favor liquid production. |
| Temperature | 400-550°C | 'Sweet spot' for decomposing biomass into liquids, not gases. |
| Vapor Residence Time | < 2 seconds | Prevents vapor breakdown into gas, maximizing bio-oil yield. |
| Primary Product | Bio-oil | Liquid fuel and chemical feedstock. |
| Key Co-product | Biochar | Solid residue used for soil amendment or activated carbon. |
Ready to Optimize Your Biomass Conversion Process?
Whether your goal is to produce bio-oil, biochar, or syngas, the right laboratory equipment is critical for research and development. KINTEK specializes in providing high-quality lab reactors, furnaces, and pyrolysis systems tailored to your specific thermal conversion needs.
We help you:
- Test and optimize pyrolysis parameters for maximum yield.
- Scale up your process from lab to pilot with reliable equipment.
- Achieve precise control over temperature, heating rate, and residence time.
Let's discuss your project. Contact our experts today to find the perfect laboratory solution for your biomass conversion challenges.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















