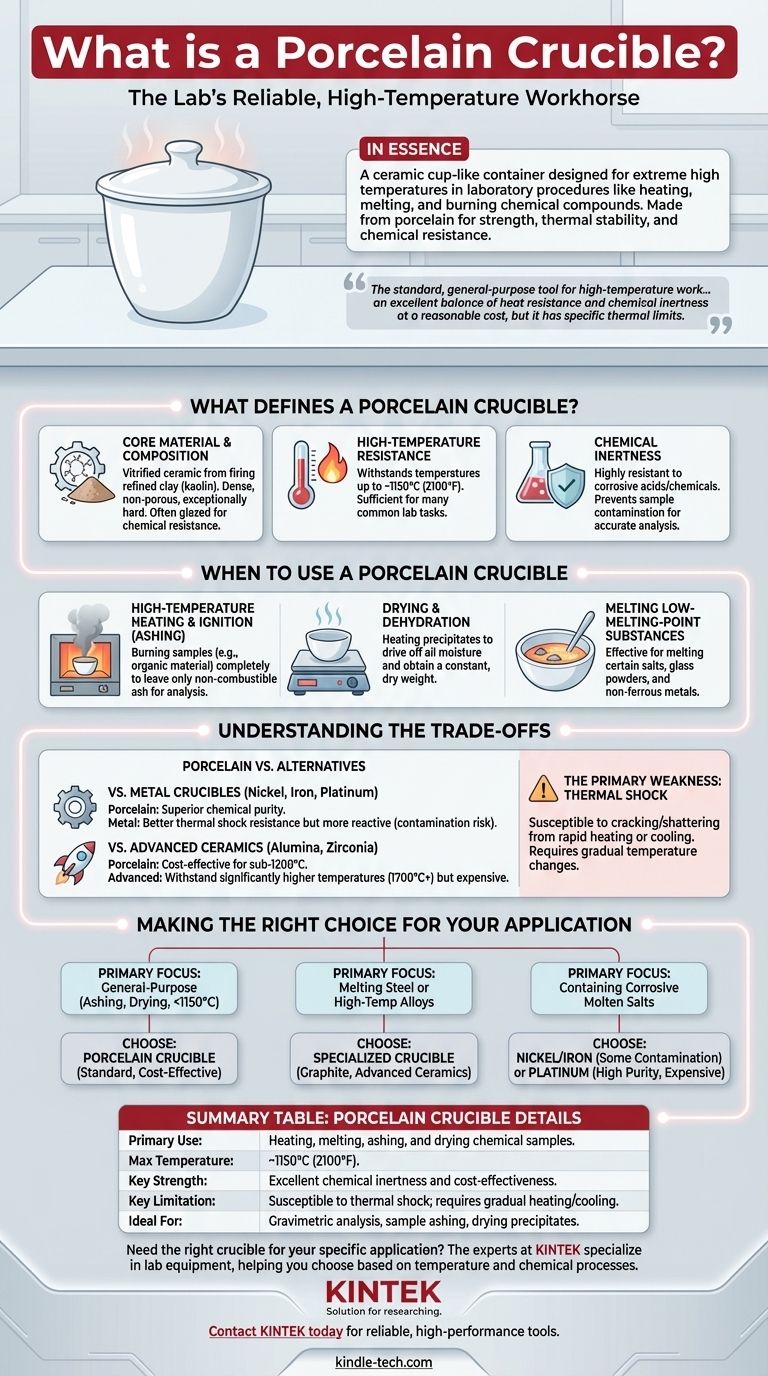
In essence, a porcelain crucible is a ceramic cup-like container designed to withstand extremely high temperatures for laboratory procedures. It is used for tasks like heating, melting, and burning chemical compounds and samples. Its primary material is porcelain, a specific type of ceramic known for its strength, thermal stability, and chemical resistance.
A porcelain crucible is the standard, general-purpose tool for high-temperature work in a lab. It offers an excellent balance of heat resistance and chemical inertness at a reasonable cost, but it is not indestructible and has specific thermal limits you must respect.

What Defines a Porcelain Crucible?
A crucible is simply a container for high-temperature work. The material it's made from dictates its specific capabilities and limitations. Porcelain is a common and reliable choice for many applications.
Core Material and Composition
Porcelain is a type of vitrified (glass-like) ceramic. It is produced by firing a refined clay, typically kaolin, at very high temperatures. This process results in a material that is dense, non-porous, and exceptionally hard.
Most laboratory-grade porcelain crucibles are also glazed on the interior and exterior (except for the bottom surface) to increase their resistance to chemical attack and make them easier to clean.
Key Property: High-Temperature Resistance
The primary function of a porcelain crucible is to endure intense heat without melting, breaking, or reacting with its contents.
Porcelain can typically withstand temperatures up to approximately 1150°C (2100°F). While this is lower than specialized ceramics like alumina or zirconia, it is more than sufficient for many common laboratory tasks.
Key Property: Chemical Inertness
Porcelain is highly resistant to the corrosive action of most acids and other chemicals, even at high temperatures. This ensures that the crucible itself does not contaminate the sample being heated, which is critical for accurate analytical chemistry.
When to Use a Porcelain Crucible
Porcelain crucibles are a workhorse in chemistry labs, particularly for gravimetric analysis, where precise mass measurements before and after heating are required.
High-Temperature Heating and Ignition
The most common use is for the ashing of samples. This involves heating a substance, such as a filter paper or organic material, at a high temperature until it burns away completely, leaving only a non-combustible ash or residue for analysis.
Drying and Dehydration
Crucibles are used to heat chemical precipitates to drive off all moisture and obtain a constant, dry weight. The non-porous surface prevents absorption of water that could alter the final measurement.
Melting Low-Melting-Point Substances
While not suitable for high-temperature steel or exotic alloys, porcelain is effective for melting certain salts, glass powders, and non-ferrous metals with lower melting points.
Understanding the Trade-offs
Choosing a porcelain crucible means accepting a specific set of strengths and weaknesses. It is not always the best tool for every high-temperature job.
Compared to Metal Crucibles
Metal crucibles (made of nickel, iron, or platinum) can often handle thermal shock better. However, they are far more chemically reactive and can easily contaminate a sample, especially under acidic conditions. Porcelain is chosen when chemical purity is paramount.
Compared to Advanced Ceramics
Specialized ceramics like alumina, zirconia, and silicon carbide can withstand significantly higher temperatures (1700°C and above). However, these materials are substantially more expensive. Porcelain offers a cost-effective solution for the vast majority of sub-1200°C applications.
The Primary Weakness: Thermal Shock
The most significant limitation of porcelain is its susceptibility to thermal shock. Heating or cooling the crucible too rapidly will create internal stresses, causing it to crack or shatter. It must always be heated and cooled gradually to ensure its longevity.
Making the Right Choice for Your Application
Selecting the correct crucible material is critical for the success and safety of your work.
- If your primary focus is general-purpose ashing, drying precipitates, or heating below 1150°C: A porcelain crucible is the standard, most cost-effective choice.
- If your primary focus is melting steel or other high-temperature alloys: You must use a specialized crucible made of graphite, clay-graphite, or advanced ceramics.
- If your primary focus is containing highly corrosive molten salts (alkaline fluxes): A nickel or iron crucible may be more suitable, though it will contaminate the sample. For analytical purity, an expensive platinum crucible is required.
Understanding the properties of your tools is the first step toward achieving reliable and accurate results in the lab.
Summary Table:
| Property | Porcelain Crucible Details |
|---|---|
| Primary Use | Heating, melting, ashing, and drying chemical samples |
| Max Temperature | ~1150°C (2100°F) |
| Key Strength | Excellent chemical inertness and cost-effectiveness |
| Key Limitation | Susceptible to thermal shock; requires gradual heating/cooling |
| Ideal For | Gravimetric analysis, sample ashing, drying precipitates |
Need the right crucible for your specific application?
Porcelain crucibles are a lab staple, but selecting the correct equipment is crucial for safety and accuracy. The experts at KINTEK specialize in lab equipment and consumables, serving all your laboratory needs. We can help you choose the perfect crucible—whether porcelain, advanced ceramic, or metal—based on your temperature requirements and chemical processes.
Contact KINTEK today to ensure your lab has the reliable, high-performance tools it needs for precise results.
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- Why is a clay graphite crucible preferred for the preparation of Al-1wt.%Fe-1wt.%Ni alloy solutions? Key Benefits Explained
- What can be used as a crucible? Match the Right Material to Your Melting Needs
- What is the primary function of high-purity alumina crucibles in LBE corrosion experiments? Ensure Data Integrity
- Why are alumina crucibles selected for LTPO synthesis? Ensure Chemical Purity in High-Temperature Calcination
- What is the function of ceramic crucibles during the industrial chemical analysis of charcoal? Enhance Data Accuracy
- Why is the choice of crucible material critical for liquid lead corrosion? Ensure High-Purity Experimental Integrity
- Why do my crucibles keep breaking? Prevent Thermal Shock and Extend Crucible Life
- Why are Pt-Rh crucibles used for aluminoborosilicate glass? Ensure Maximum Purity at 1450°C



















