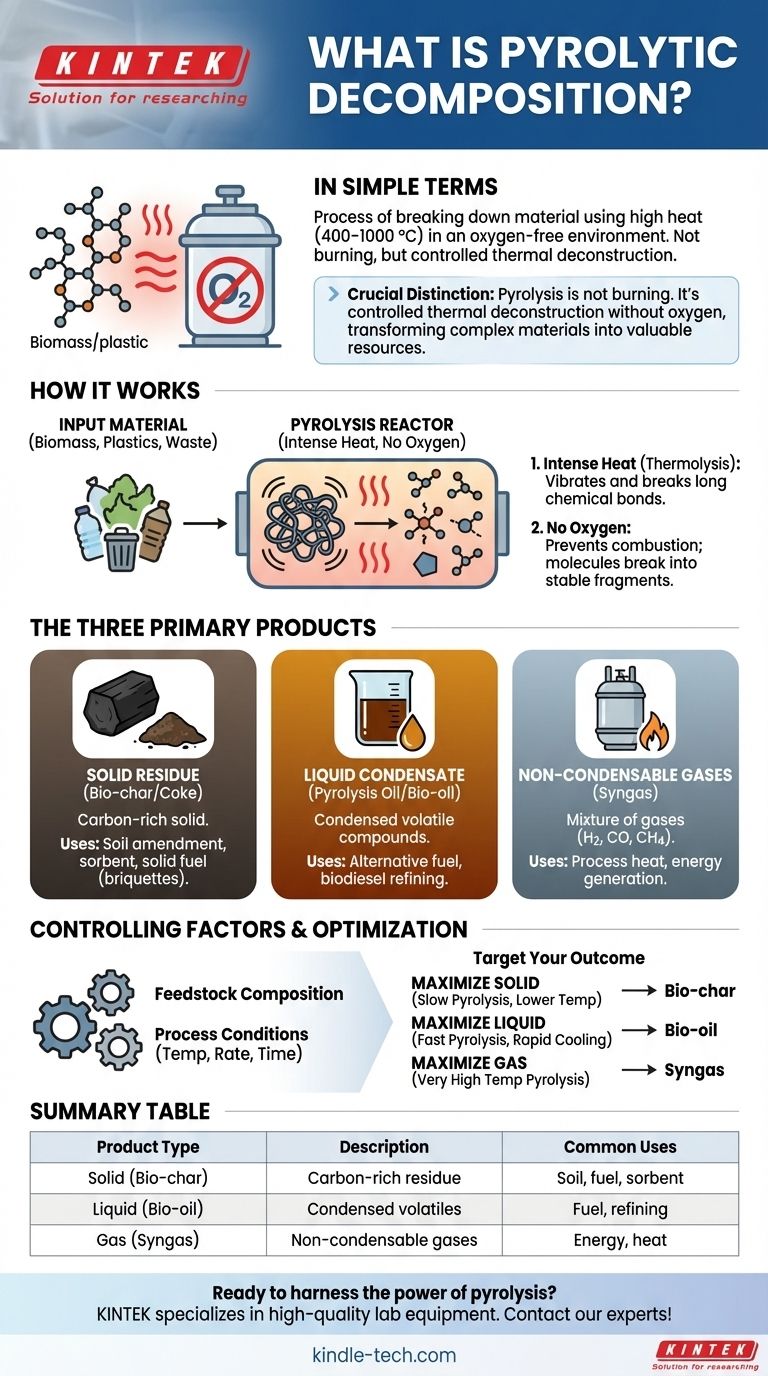
In simple terms, pyrolytic decomposition, or pyrolysis, is the process of breaking down a material using high heat in an environment without oxygen. Instead of burning, the substance's chemical bonds are fractured by the intense thermal energy (typically 400–1000 °C), causing it to decompose into simpler, more stable components. This technique is commonly used on complex, high-molecular-weight materials like biomass, plastics, or waste.
The crucial distinction to understand is that pyrolysis is not burning; it's a controlled thermal deconstruction. By preventing combustion with an oxygen-free environment, you can transform complex materials into a predictable set of valuable solid, liquid, and gaseous products.

How Pyrolytic Decomposition Works
Pyrolysis is a powerful thermochemical process driven by two fundamental conditions: intense heat and the absence of an oxidizer like oxygen.
The Role of High Heat
The core mechanism of pyrolysis is the application of high temperatures. This thermal energy is strong enough to vibrate and break the long, complex chemical bonds within the original material, a process known as thermolysis.
The Critical Absence of Oxygen
This is what separates pyrolysis from combustion. Without oxygen, the material cannot "burn" in the traditional sense. Instead of reacting with oxygen to produce fire, ash, and smoke, the molecules simply break apart into smaller, more stable fragments.
Breaking Down Complex Molecules
This method is particularly effective for organic materials or polymers with very high molecular weights. These large, cumbersome molecules are deconstructed into a mixture of simpler, smaller molecules, which can then be collected as distinct products.
The Three Primary Products of Pyrolysis
The output of pyrolysis is not a single substance but a mixture of solids, liquids, and gases. The exact proportions depend heavily on the input material and the specific process conditions.
Solid Residue (Bio-char or Coke)
This is the carbon-rich solid material left behind after the volatile components have been driven off. It is a stable product with uses in agriculture (as a soil amendment), as an industrial sorbent, or as a solid fuel source (briquettes).
Liquid Condensate (Pyrolysis Oil or Bio-oil)
After the volatile gases are created, they can be cooled and condensed into a liquid. This pyrolysis oil is a complex mixture of compounds that can be used as an alternative fuel or further refined into higher-value products like biodiesel.
Non-Condensable Gases (Syngas)
This is a mixture of gases (like hydrogen, carbon monoxide, and methane) that do not condense back into a liquid upon cooling. This "syngas" has fuel value and is often captured and used to provide the heat energy for the pyrolysis process itself, making it partially self-sustaining.
Understanding the Controlling Factors
While the process is straightforward in principle, the results can be highly variable. Controlling the outcome requires a precise understanding of key factors.
Feedstock Composition is Key
The single biggest factor determining the output is the input material. The pyrolysis of wood will produce very different oils, gases, and char compared to the pyrolysis of waste plastic or tires.
Process Conditions Dictate Yields
How the heat is applied matters immensely. Temperature, heating rate, and the amount of time the material spends at that temperature will shift the balance of the final products.
For example, slow pyrolysis at lower temperatures tends to maximize the yield of solid bio-char. In contrast, fast pyrolysis followed by rapid quenching (cooling) is used to maximize the yield of liquid bio-oil.
Making the Right Choice for Your Goal
The versatility of pyrolysis means it can be tailored to achieve different outcomes. The optimal approach depends entirely on which end product you value most.
- If your primary focus is creating a soil amendment or solid carbon: Use slow, lower-temperature pyrolysis to maximize the yield of solid bio-char.
- If your primary focus is producing liquid fuel: Use fast pyrolysis with rapid cooling to maximize the collection and preservation of bio-oil.
- If your primary focus is generating energy or synthesis gas: Use very high-temperature pyrolysis to maximize the conversion of the material into non-condensable gas.
Ultimately, pyrolytic decomposition serves as a powerful and flexible tool for transforming low-value feedstocks into a range of valuable resources.
Summary Table:
| Product Type | Description | Common Uses |
|---|---|---|
| Solid (Bio-char/Coke) | Carbon-rich solid residue | Soil amendment, sorbent, solid fuel |
| Liquid (Bio-oil) | Condensed volatile compounds | Alternative fuel, biodiesel refining |
| Gas (Syngas) | Non-condensable gases (H2, CO, CH4) | Process heat, energy generation |
Ready to harness the power of pyrolysis in your lab? KINTEK specializes in high-quality lab equipment, including pyrolysis reactors and ovens, to help you efficiently convert biomass, plastics, and other materials into valuable products like bio-oil and bio-char. Contact our experts today to find the perfect pyrolysis solution for your research or processing needs!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Lab-Scale Vacuum Induction Melting Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What defines the cascading and centrifuging modes of bed motion in a rotary kiln? Master Kiln Speed for Peak Performance
- What are the distinct regions within the material bed during the rolling mode of motion? Optimize Mixing Efficiency
- How do you rejuvenate activated carbon? Restoring Adsorption Power with Thermal Reactivation
- What is a rotary kiln electric furnace? Achieve Superior Uniform Heating for Your Materials
- What is pyrolysis decomposition of biomass? Unlock Value from Organic Waste
- What is the feedstock for biochar? A Guide to Choosing the Right Biomass for Your Needs
- What are the advantages of pyrolysis in waste management? Turn Waste into Fuel and Valuable Resources
- What size is a rotary kiln? A Custom Solution for Your Process Needs



















