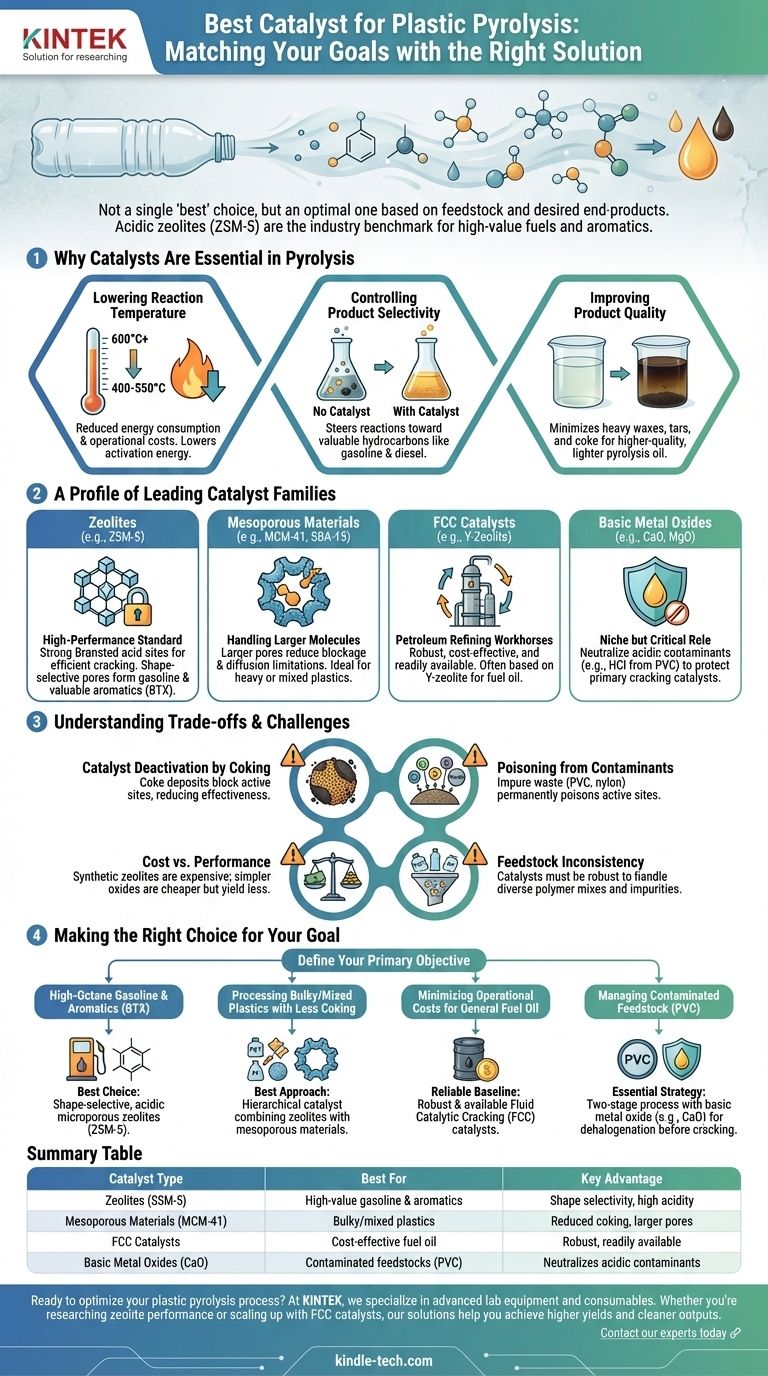
The "best" catalyst for plastic pyrolysis is not a single substance, but rather a choice dictated by your specific goals. The optimal catalyst depends entirely on the type of plastic feedstock being processed and the desired chemical end-products. However, for producing high-value liquid fuels and aromatic chemicals from common plastics like polyolefins, acidic zeolite catalysts, particularly ZSM-5, are widely recognized as the industry benchmark due to their exceptional performance and selectivity.
The central challenge is not finding a single "best" catalyst, but rather matching a catalyst's specific properties—such as acidity, pore size, and stability—to your unique plastic waste stream and target financial outcomes.

Why Catalysts Are Essential in Pyrolysis
Catalytic pyrolysis represents a significant advancement over thermal pyrolysis alone. The introduction of a catalyst fundamentally transforms the process, offering critical operational and economic advantages.
Lowering Reaction Temperature
Catalysts create an alternative reaction pathway with a lower activation energy. This allows the complex polymer chains in plastic to break down at significantly lower temperatures (e.g., 400-550°C) compared to non-catalytic processes (often >600°C), resulting in substantial energy savings.
Controlling Product Selectivity
Without a catalyst, pyrolysis produces a broad, difficult-to-refine mixture of gases, liquids (pyrolysis oil), and solid char. Catalysts steer the chemical reactions toward specific, more valuable products, such as hydrocarbons within the gasoline or diesel range.
Improving Product Quality
A well-chosen catalyst can minimize the formation of undesirable heavy waxes, tars, and coke (solid carbon residue). This leads to a higher-quality, lighter pyrolysis oil that is easier to upgrade and process downstream.
A Profile of Leading Catalyst Families
Different families of catalysts offer distinct advantages based on their structure and chemical properties. The choice among them is a foundational engineering decision.
Zeolites: The High-Performance Standard
Zeolites are crystalline aluminosilicates with a highly defined, microporous structure. Their effectiveness stems from their strong Brønsted acid sites, which are exceptionally efficient at cracking long polymer chains.
ZSM-5 is the most prominent example. Its unique intersecting pore channel system (around 5.5 Å) provides shape selectivity, meaning it preferentially forms molecules that can fit within and diffuse out of its pores, like gasoline-range hydrocarbons and valuable aromatics (benzene, toluene, xylene).
Mesoporous Materials: Handling Larger Molecules
While zeolites are highly effective, their small pores can become blocked by bulky plastic molecules or coke deposits. Mesoporous materials like MCM-41 and SBA-15 have much larger pore diameters (2-50 nm).
These materials can accommodate larger polymer fragments, reducing diffusion limitations and making them more resistant to deactivation when processing heavy or mixed plastics. They are often used in conjunction with zeolites to create a hierarchical system.
Fluid Catalytic Cracking (FCC) Catalysts
These are the workhorses of the petroleum refining industry, designed for cracking long-chain hydrocarbons into gasoline. Commercial FCC catalysts are robust, well-understood, and often based on Y-zeolite.
Because they are produced at a massive scale, FCC catalysts are a cost-effective and readily available option that can be directly applied or adapted for plastic pyrolysis.
Basic Metal Oxides: A Niche but Critical Role
Inexpensive metal oxides like calcium oxide (CaO) or magnesium oxide (MgO) play a different role. They are not primarily used for cracking but are excellent for neutralizing acidic contaminants.
When processing plastics like PVC, which releases corrosive hydrochloric acid (HCl), these basic oxides can be used in a pre-treatment step or mixed in to capture the contaminants and protect the primary cracking catalyst from being poisoned.
Understanding the Trade-offs and Challenges
No catalyst is a perfect solution. An effective design requires acknowledging and mitigating several key operational challenges.
Catalyst Deactivation by Coking
The most common issue is the formation of coke—a carbonaceous deposit—on the catalyst's surface and within its pores. This deposit physically blocks the active sites where reactions occur, rapidly reducing the catalyst's effectiveness over time.
Poisoning from Contaminants
Real-world plastic waste is never pure. Contaminants like chlorine (from PVC), nitrogen (from nylon), sulfur, and various metals can chemically bond to the catalyst's active sites, permanently poisoning and deactivating them.
Cost vs. Performance
There is a direct trade-off between catalyst cost and performance. Highly engineered synthetic zeolites can be expensive, while simpler amorphous silica-alumina or basic metal oxides are cheaper but may offer lower yields of the most valuable products.
Feedstock Inconsistency
The variable nature of municipal plastic waste means a catalyst must be robust enough to handle a mix of polymer types and impurities. A catalyst optimized for pure polyethylene may perform poorly with a PET-contaminated stream.
Making the Right Choice for Your Goal
Selecting the best catalyst begins with defining your primary objective. Different goals demand different catalytic strategies.
- If your primary focus is high-octane gasoline and aromatics (BTX): Shape-selective, highly acidic microporous zeolites like ZSM-5 are the undisputed best choice for this application.
- If your primary focus is processing bulky or mixed plastics with less coking: A hierarchical catalyst combining the activity of zeolites with the superior mass transfer of mesoporous materials is the most effective approach.
- If your primary focus is minimizing operational costs for general fuel oil production: Commercially available and robust Fluid Catalytic Cracking (FCC) catalysts provide a reliable, cost-effective baseline.
- If your primary focus is managing contaminated feedstock containing PVC: A two-stage process using a basic metal oxide (like CaO) for dehalogenation followed by a cracking catalyst is essential to ensure system longevity.
Ultimately, the most effective pyrolysis process is built on a clear understanding of your goals and a catalyst strategy tailored to meet them.
Summary Table:
| Catalyst Type | Best For | Key Advantage |
|---|---|---|
| Zeolites (e.g., ZSM-5) | High-value gasoline & aromatics | Shape selectivity, high acidity |
| Mesoporous Materials (e.g., MCM-41) | Bulky/mixed plastics | Reduced coking, larger pores |
| FCC Catalysts | Cost-effective fuel oil | Robust, readily available |
| Basic Metal Oxides (e.g., CaO) | Contaminated feedstocks (e.g., PVC) | Neutralizes acidic contaminants |
Ready to optimize your plastic pyrolysis process? At KINTEK, we specialize in providing advanced lab equipment and consumables tailored to your catalytic pyrolysis needs. Whether you're researching zeolite performance or scaling up with FCC catalysts, our solutions help you achieve higher yields and cleaner outputs. Contact our experts today to discuss how we can support your laboratory's innovation in waste-to-energy conversion!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Custom PTFE Teflon Parts Manufacturer for PTFE Buchner Funnel and Triangular Funnel
- Custom PTFE Teflon Parts Manufacturer for Magnetic Stirring Bar
- Bomb Type Probe for Steelmaking Production Process
People Also Ask
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- What are the specific applications of PTFE in micro-batch slug flow systems? Enhance Your Microfluidic Reaction Purity
- What is the function of PTFE reaction kettle bodies in micro-CSTR systems? Enhance Chemical Stability & Flow
- Why is PTFE wire used for hanging metal specimens in biodiesel corrosion tests? Ensure Pure Experimental Results
- What are alloys in simple words? Unlock the Power of Engineered Materials



















