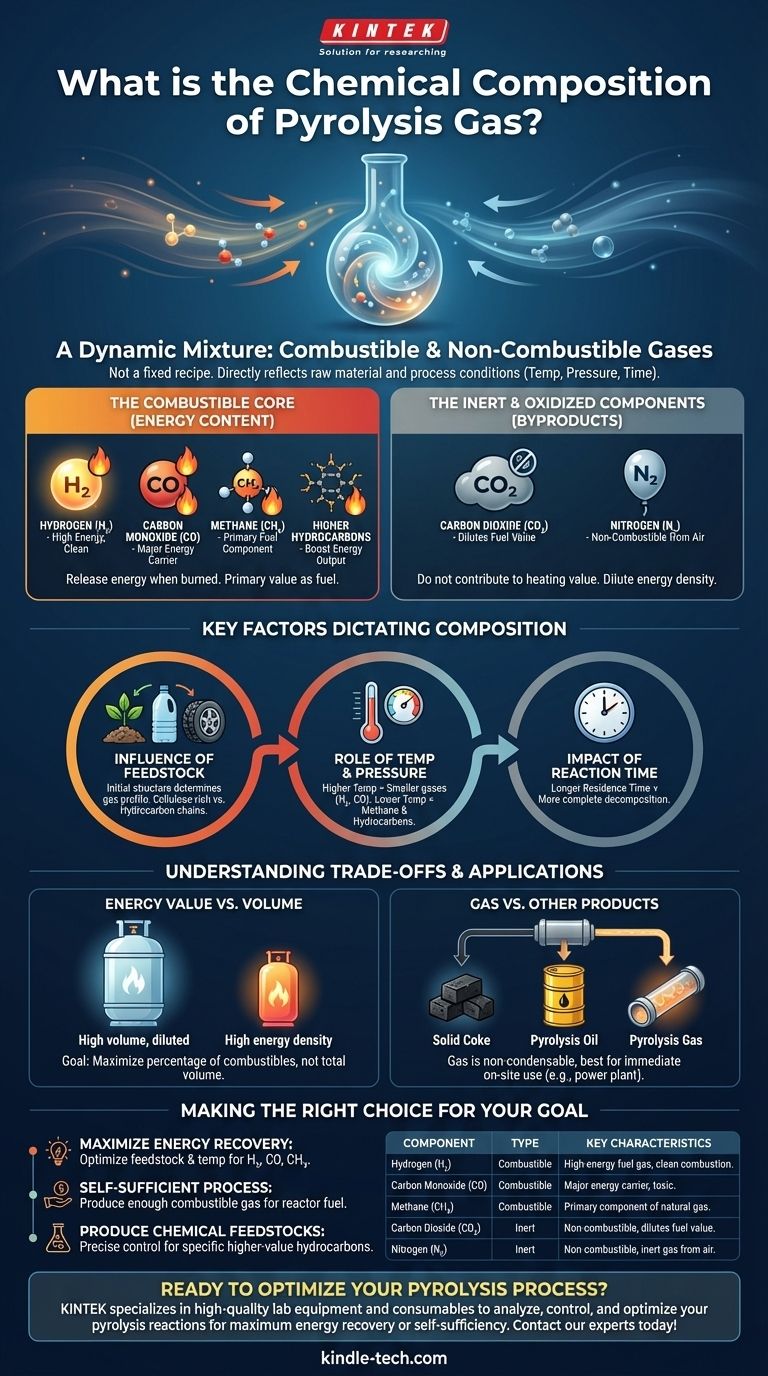
At its core, pyrolysis gas is a dynamic mixture of combustible and non-combustible gases. This non-condensable gas primarily consists of carbon monoxide (CO), hydrogen (H₂), methane (CH₄), and carbon dioxide (CO₂). The mixture also contains nitrogen (N₂) and other higher-value hydrocarbons, with the exact proportions varying significantly based on the production process.
The specific chemical makeup of pyrolysis gas is not a fixed recipe. It is a direct reflection of the raw material being processed and the precise conditions—temperature, pressure, and time—under which the pyrolysis takes place.

Deconstructing the Components of Pyrolysis Gas
To understand pyrolysis gas, we must separate its components into two functional categories: those that provide energy and those that are inert byproducts.
The Combustible Core (Energy Content)
The value of pyrolysis gas as a fuel comes from its combustible components. These are the gases that release energy when burned.
The primary energy carriers are hydrogen (H₂), carbon monoxide (CO), and methane (CH₄). The presence of other, more complex hydrocarbons (like ethane or propane) further increases its potential energy output.
The Inert and Oxidized Components
Not all gases in the mixture contribute to its heating value. These components are byproducts of the chemical decomposition that occurs during pyrolysis.
Carbon dioxide (CO₂) and nitrogen (N₂) are the main non-combustible gases. While they are a natural part of the output, a higher concentration of these gases dilutes the fuel, lowering its overall energy density.
Why Pyrolysis Gas Composition Is Never Fixed
The composition of pyrolysis gas is highly variable because it is an output, not an input. Three key factors dictate the final chemical mix.
The Influence of Feedstock
The initial chemical structure of the raw material is the most important variable. A feedstock rich in cellulose and hemicellulose (like biomass) will produce a different gas profile than one based on long hydrocarbon chains (like plastics or tires).
The Role of Temperature and Pressure
Temperature has a direct impact on the chemical breakdown. Higher temperatures tend to crack larger organic molecules into smaller, simpler gases like hydrogen and carbon monoxide. Lower temperatures may result in a higher concentration of methane and other hydrocarbons.
The Impact of Reaction Time
The duration the feedstock is exposed to the pyrolysis conditions, known as residence time, also influences the final gas composition. A longer time allows for more complete decomposition into the simplest gas molecules.
Understanding the Trade-offs
Using or analyzing pyrolysis gas requires understanding its inherent limitations and how it compares to other outputs.
Energy Value vs. Volume
A pyrolysis process might produce a large volume of gas, but if it is heavily diluted with CO₂ and N₂, its practical heating value may be quite low. The goal is often to maximize the percentage of combustible components, not just the total gas output.
Pyrolysis Gas vs. Other Products
Pyrolysis produces three primary products: solid coke, liquid pyrolysis oil, and the gas itself. The gas is non-condensable and difficult to store, making it ideal for immediate, on-site use. Pyrolysis oil, a liquid, can be more easily stored, transported, and refined.
The Goal of Self-Sufficiency
Because its energy density can be variable, the most common and efficient use for pyrolysis gas is to power the pyrolysis plant itself. It is burned to generate the heat needed to sustain the reaction, creating a closed-loop, self-sufficient energy system.
Making the Right Choice for Your Goal
The optimal composition of pyrolysis gas depends entirely on its intended application.
- If your primary focus is maximizing energy recovery: You must optimize feedstock and temperature to increase the percentage of combustible gases like H₂, CO, and CH₄.
- If your primary focus is creating a self-sufficient process: The key is simply to produce enough combustible gas, regardless of its specific composition, to consistently fuel the pyrolysis reactor.
- If your primary focus is producing chemical feedstocks: You need precise control over all process parameters to favor the creation of specific higher-value hydrocarbons over simple fuel gases.
Understanding the variables that shape pyrolysis gas composition is the first step toward controlling the process to achieve a specific outcome.
Summary Table:
| Component | Type | Key Characteristics |
|---|---|---|
| Hydrogen (H₂) | Combustible | High-energy fuel gas, clean combustion. |
| Carbon Monoxide (CO) | Combustible | Major energy carrier, toxic. |
| Methane (CH₄) | Combustible | Primary component of natural gas. |
| Carbon Dioxide (CO₂) | Inert | Non-combustible, dilutes fuel value. |
| Nitrogen (N₂) | Inert | Non-combustible, inert gas from air. |
Ready to optimize your pyrolysis process for maximum energy recovery or self-sufficiency?
The precise composition of your pyrolysis gas is critical to your project's success. At KINTEK, we specialize in providing the high-quality lab equipment and consumables you need to analyze, control, and optimize your pyrolysis reactions.
Whether you're processing biomass, plastics, or other feedstocks, our solutions help you achieve the gas composition you need for your specific goals. Contact our experts today to discuss how we can support your laboratory's pyrolysis research and development.
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Double Plate Heating Press Mold for Lab
- Lab Sterile Slapping Type Homogenizer for Tissue Mashing and Dispersing
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Custom PTFE Teflon Parts Manufacturer PTFE Beaker and Lids
People Also Ask
- Why is a precision vibratory sieving system important for Pt/Pd alloy analysis? Ensure Data Integrity & XRD Accuracy
- What are the disadvantages of sieve machine? Key Limitations in Particle Size Analysis
- How do high-precision sieving systems benefit zeolite preparation? Maximize Adsorption for Wastewater Treatment
- Why is a standardized sieving system necessary for elephant grass research? Ensure Reliable Sample Consistency
- Why is a laboratory electromagnetic vibratory sieve shaker used? Optimize Walnut Shell Chemical Pretreatment















