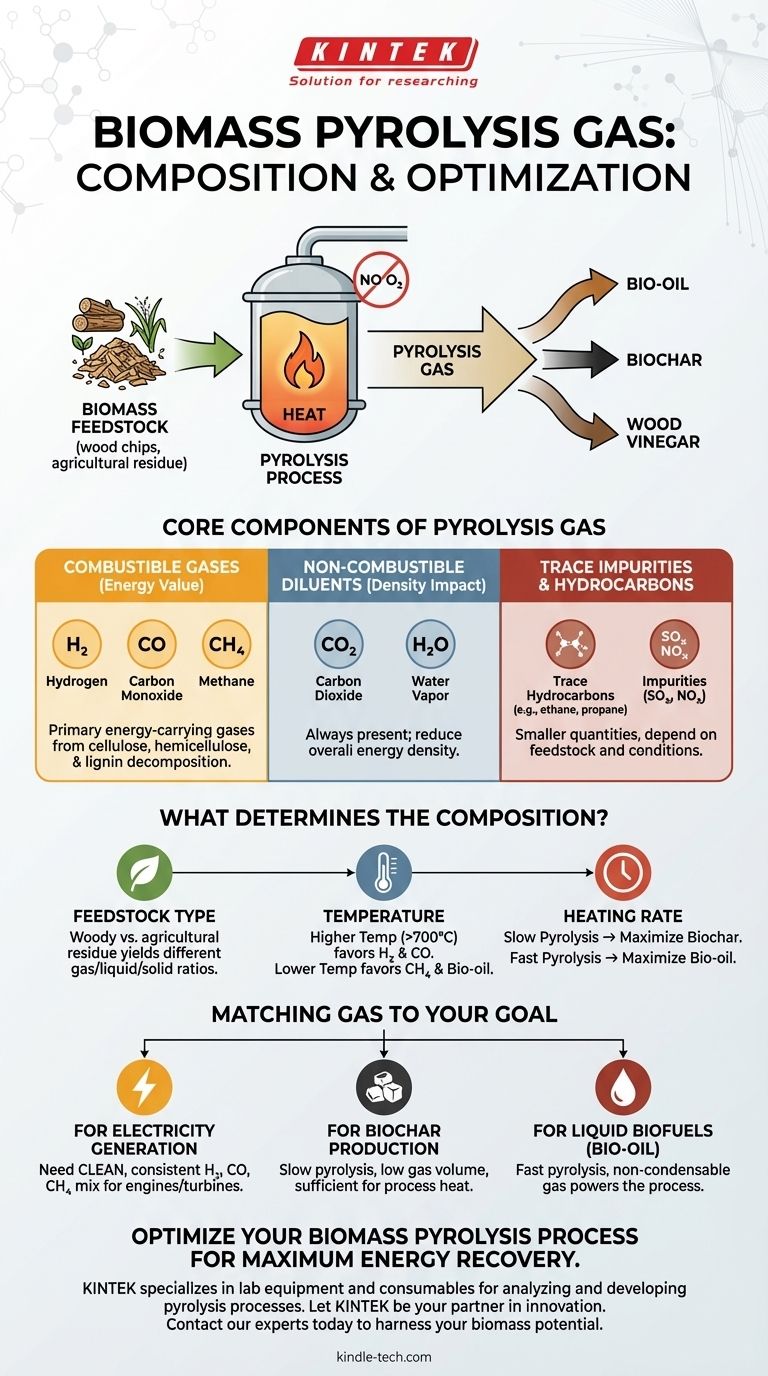
The gas produced during biomass pyrolysis is a mixture of combustible and non-combustible components. The primary valuable, energy-carrying gases are carbon monoxide (CO), hydrogen (H₂), and methane (CH₄), which are generated alongside non-combustible gases like carbon dioxide (CO₂) and trace amounts of other light hydrocarbons. This gas is one of several co-products created during the process, which also yields bio-oil, biochar, and wood vinegar.
The specific composition of pyrolysis gas is not a fixed recipe; it is a direct outcome of the original biomass feedstock and the precise conditions of the pyrolysis process, particularly temperature. Understanding these variables is key to controlling the gas's energy content and suitability for its intended use.

The Core Components of Pyrolysis Gas
Pyrolysis gas, often called "syngas" in a related gasification context, is the non-condensable fraction produced when biomass is heated in the absence of oxygen. Its composition can be broken down into three main categories.
The Combustible Gases
These components are what give the gas its energy value. They are the direct result of the thermal decomposition of the cellulose, hemicellulose, and lignin that make up the biomass.
The primary combustible gases are:
- Hydrogen (H₂)
- Carbon Monoxide (CO)
- Methane (CH₄)
Non-Combustible Diluents
These gases do not contribute to the heating value but are always present in the mixture. Their concentration impacts the overall energy density of the gas.
The main non-combustible components include:
- Carbon Dioxide (CO₂)
- Water Vapor (H₂O)
Trace Hydrocarbons and Impurities
Depending on the feedstock and process conditions, smaller quantities of other light hydrocarbon gases (like ethane and propane) and potential impurities (like low levels of SOx and NOx) may also be present.
What Determines the Final Gas Composition?
You cannot view pyrolysis gas as a single, uniform product. Its final makeup is highly dependent on several key operational factors, making the process both a challenge and an opportunity for optimization.
The Influence of Feedstock
The type of biomass used is the starting point. A woody biomass with high lignin content will break down differently than an agricultural residue with high cellulose content, yielding different ratios of gas, liquid, and solid products.
The Critical Role of Temperature
Temperature is arguably the most significant control lever. Higher process temperatures (e.g., >700°C) tend to favor the production of hydrogen and carbon monoxide, promoting further cracking of heavier tars into lighter gases. Lower temperatures often result in a higher yield of methane and condensable liquids (bio-oil).
The Impact of Heating Rate
The speed at which biomass is heated also dictates the final product distribution.
- Slow Pyrolysis: Longer residence times and slow heating are used to maximize the solid biochar yield. The gas produced is often a lower-volume co-product used to supply heat to the reactor.
- Fast Pyrolysis: Very rapid heating and short residence times are designed to maximize the liquid bio-oil yield. The gas produced in this scenario is the fraction that doesn't condense and is also typically used to power the process.
Understanding the Trade-offs
Optimizing for one output of pyrolysis inevitably means compromising on another. The gas composition is directly tied to these production choices.
Energy Content vs. Purity
A gas stream with a high concentration of methane (CH₄) will have a higher calorific value than one dominated by CO and H₂. However, the raw gas from a reactor is never pure; it is mixed with aerosols of tar and wood vinegar that must be cleaned or "conditioned" before the gas can be used in sensitive equipment like an engine.
Gas Yield vs. Other Products
The goal of most pyrolysis operations is to produce either high-value biochar or bio-oil. In these cases, the gas is a secondary product whose primary role is to provide the energy needed to make the process self-sustaining. Its composition is a byproduct of the conditions chosen to optimize for the other outputs.
Process Complexity
Achieving a specific, high-quality gas composition often requires more advanced reactor designs and tighter control over process parameters. This increases capital and operational costs, which must be justified by the value of the end product.
Matching the Gas to Your Goal
The "ideal" gas composition depends entirely on your end-use application. Your operational strategy should be aligned with this goal from the start.
- If your primary focus is generating electricity: You need a clean, consistent gas stream. The priority is stable combustion in an engine or turbine, making a reliable mix of H₂, CO, and CH₄ crucial after sufficient tar removal.
- If your primary focus is producing biochar: You will be using slow pyrolysis. The resulting gas stream will likely be low in volume but sufficient to heat your reactor, making its exact composition less critical than its ability to sustain the process.
- If your primary focus is creating liquid biofuels (bio-oil): You will use fast pyrolysis. The non-condensable gas fraction is simply the fuel source that powers the high-energy demands of the process.
Ultimately, controlling the pyrolysis process allows you to tailor the gas composition to meet your specific energy or product objectives.
Summary Table:
| Component | Type | Key Characteristics |
|---|---|---|
| Hydrogen (H₂) | Combustible | High-energy gas, production favored at high temperatures. |
| Carbon Monoxide (CO) | Combustible | Major energy carrier, also produced more at high temperatures. |
| Methane (CH₄) | Combustible | High calorific value, more common at lower pyrolysis temperatures. |
| Carbon Dioxide (CO₂) | Non-Combustible | Diluent that lowers the overall energy density of the gas. |
| Water Vapor (H₂O) | Non-Combustible | Present from moisture in the feedstock and as a reaction product. |
| Trace Hydrocarbons | Combustible | Minor components like ethane and propane; vary with process conditions. |
Ready to optimize your biomass pyrolysis process for maximum energy recovery?
Understanding and controlling pyrolysis gas composition is critical for achieving your project's goals, whether for power generation, process heat, or co-product optimization. KINTEK specializes in providing robust lab equipment and consumables for analyzing and developing pyrolysis processes. Our expertise helps you precisely characterize your gas output and tailor your setup for efficiency and performance.
Let KINTEK be your partner in innovation. Contact our experts today to discuss how our solutions can help you harness the full potential of your biomass resources.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Vibratory Sieve Shaker Machine Slap Vibrating Sieve
People Also Ask
- What are the negative effects of pyrolysis? High Costs and Environmental Risks Explained
- What are the results of calcination? A Guide to Purification and Material Transformation
- What is spray pyrolysis method? A Guide to Precision Thin Film & Powder Synthesis
- How does a tilting furnace work? A Guide to Hydraulic & Mechanical Pouring Systems
- What is the process of pyrolysis and combustion? A Guide to Thermal Decomposition vs. Burning
- What can you do with pyrolysis oil? Turn Waste into Power, Fuel, and Chemicals
- What are the weaknesses of pyrolysis? Navigating High Costs and Operational Hurdles
- What is the yield of biochar in slow pyrolysis? Maximize Your Output Up to 30%

















