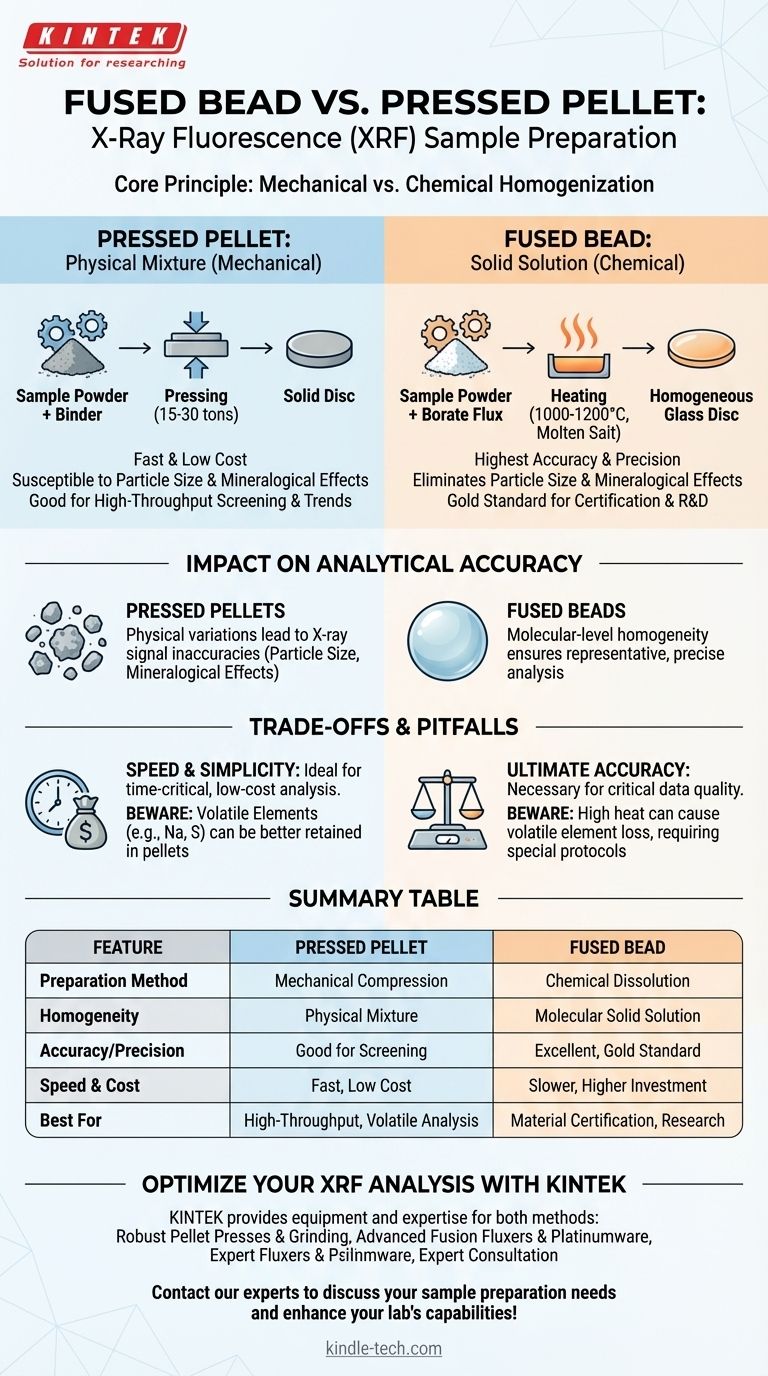
When preparing samples for X-Ray Fluorescence (XRF) analysis, the fundamental difference between a fused bead and a pressed pellet lies in the preparation method and its effect on the sample's physical state. A pressed pellet is created through a mechanical process of compacting a fine powder under high pressure. In contrast, a fused bead is formed through a chemical process where the sample is dissolved in a molten salt (a flux) and cooled into a perfectly homogeneous glass disc.
The choice between a pressed pellet and a fused bead is a fundamental trade-off between speed and cost on one side, and analytical accuracy and reliability on the other. Pressed pellets are fast and inexpensive, while fused beads provide the highest possible accuracy by eliminating physical sample variations.

The Core Principle: Mechanical vs. Chemical Homogenization
The goal of any XRF sample preparation is to present a flat, dense, and homogeneous surface to the instrument. How pellets and beads achieve this homogeneity is their primary point of divergence.
How Pressed Pellets are Made
A pressed pellet is created by first grinding a sample into a very fine, uniform powder.
This powder is often mixed with a binding agent to improve its stability. The mixture is then poured into a die and compacted under immense pressure (typically 15-30 tons) to form a solid, cohesive disc.
This is purely a physical process. The original chemical and mineralogical structures of the individual powder grains remain, just packed tightly together.
How Fused Beads are Made
Creating a fused bead is a high-temperature chemical transformation.
A precise amount of the powdered sample is mixed with a much larger amount of a borate flux, typically a lithium tetraborate/metaborate mixture. This mixture is heated in a platinum crucible to around 1000-1200°C.
At this temperature, the flux melts and acts as a powerful solvent, completely dissolving the sample material. The molten liquid is then cast into a mold and cooled to form a smooth, amorphous glass disc, which is essentially a solid solution.
The Impact on Analytical Accuracy
This difference between a physical mixture (pellet) and a solid solution (bead) has profound implications for the quality of the XRF data.
Particle Size and Mineralogical Effects in Pellets
Pressed pellets are highly susceptible to errors caused by the sample's physical characteristics.
Particle size effects occur when finer particles of one mineral and coarser particles of another segregate, leading to non-uniform X-ray absorption and fluorescence.
Mineralogical effects arise when the same element exists in different crystalline structures (e.g., iron in hematite vs. magnetite), which can affect the X-ray signal. These effects introduce inaccuracies that are difficult to correct.
The Homogeneity of Fused Beads
The fusion process completely eliminates particle size and mineralogical effects.
By dissolving the sample into a liquid state, the original physical history of the material is erased. The resulting glass bead is perfectly homogeneous on a molecular level, ensuring that the portion of the sample analyzed by the X-ray beam is truly representative of the whole.
This superior homogeneity is the primary reason why fusion produces data with significantly higher accuracy and precision.
Understanding the Trade-offs
Neither method is universally superior; the correct choice depends on your analytical goals, budget, and throughput requirements.
The Case for Pressed Pellets: Speed and Simplicity
The main advantage of pressed pellets is speed. A pellet can be prepared in just a few minutes with relatively inexpensive equipment (a grinder and a press).
This makes it the ideal method for applications where speed is more critical than absolute accuracy, such as high-throughput process control, raw material screening, or qualitative analysis.
The Case for Fused Beads: Ultimate Accuracy
Fusion is the gold standard for accuracy. While the process is slower (10-20 minutes per sample) and requires a significant capital investment in a fusion machine and platinumware, it is the only way to achieve the high-quality data required for material certification, geological research, or academic development.
Common Pitfalls to Avoid
Fusion is not without limitations. The high temperatures can cause volatile elements (like sodium, sulfur, or halogens) to be lost from the sample, skewing results. This can be mitigated with specific fluxes or capping agents, but it requires careful procedure development.
Pressed pellets avoid this issue of volatility, making them a better choice for analyzing samples where these elements are of primary interest.
Making the Right Choice for Your Goal
Select your sample preparation method based on your specific data quality requirements.
- If your primary focus is high-throughput screening or process control: Choose pressed pellets for their unmatched speed and low cost, as trend analysis is often more important than absolute accuracy.
- If your primary focus is material certification, R&D, or final quality assurance: Choose fused beads to achieve the highest possible accuracy and reliability by eliminating physical matrix effects.
- If your primary focus is analyzing for highly volatile elements: Use the pressed pellet method to avoid analyte loss, or develop a specialized fusion protocol that uses capping agents to retain the volatiles.
Ultimately, understanding this distinction empowers you to select the sample preparation method that perfectly aligns with your analytical objectives and data quality requirements.
Summary Table:
| Feature | Pressed Pellet | Fused Bead |
|---|---|---|
| Preparation Method | Mechanical compression of powder | Chemical dissolution in molten flux |
| Homogeneity | Physical mixture; susceptible to particle/mineral effects | Molecular-level solid solution; eliminates matrix effects |
| Accuracy/Precision | Good for screening and trends | Excellent; gold standard for certification/R&D |
| Speed & Cost | Fast (minutes), low equipment cost | Slower (10-20 min), higher investment (fusion machine, platinumware) |
| Best For | High-throughput control, volatile element analysis | Ultimate accuracy, material certification, research |
Optimize Your XRF Analysis with KINTEK
Choosing the right sample preparation method is critical for generating reliable, high-quality data. Whether your lab prioritizes the speed and cost-effectiveness of pressed pellets or the ultimate accuracy of fused beads, KINTEK has the equipment and expertise to support your goals.
We provide:
- Robust pellet presses and grinding equipment for efficient pressed pellet preparation.
- Advanced fusion fluxers and high-quality platinum labware for flawless fused bead creation.
- Expert consultation to help you select the ideal method for your specific materials and analytical requirements.
Let KINTEK, your trusted partner in laboratory equipment, help you achieve precise and efficient XRF analysis. Contact our experts today to discuss your sample preparation needs and enhance your lab's capabilities!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What is KBr disc method? A Complete Guide to IR Spectroscopy Sample Prep
- What role does a laboratory hydraulic press play in preparing solid model materials? Standardize for Precise Data.
- How do laboratory hydraulic presses facilitate biomass pelletization? Optimize Biofuel Density and Prevent Slagging
- How is a laboratory hydraulic press used for LLZTO pellets? Achieve 93% Density in Solid-State Battery Research
- What is the function of a laboratory hydraulic press for Li10GeP2S12 pellets? Optimize Solid-State Battery Performance



















