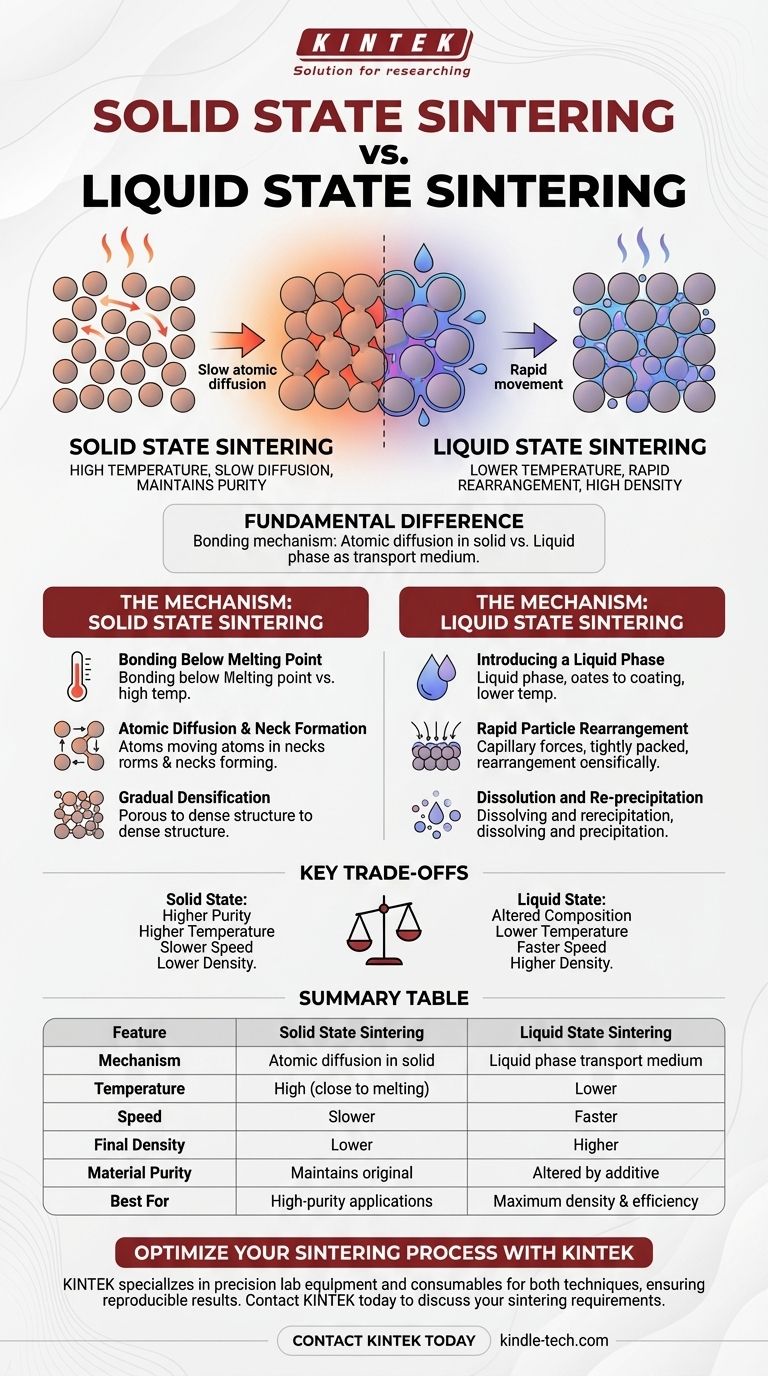
The fundamental difference between liquid state and solid state sintering lies in the mechanism used to bond powder particles together. In solid state sintering, particles are fused through atomic diffusion in a purely solid form, just below the material's melting point. Liquid state sintering introduces a small amount of a liquid phase, which acts as a transport medium to dramatically accelerate particle rearrangement and bonding.
The choice between these methods hinges on a critical trade-off: solid state sintering offers high purity at the cost of higher temperatures and longer processing times, while liquid state sintering achieves faster, lower-temperature densification by introducing a liquid that becomes part of the final material.

The Mechanism of Solid State Sintering
Solid state sintering, also known as diffusion bonding, is the most direct method for consolidating a powder into a solid mass without melting the primary material.
### Bonding Below the Melting Point
The core principle is to heat a compressed powder to a high temperature that remains below its melting point. The goal is to energize the atoms without causing a change in state.
### The Role of Atomic Diffusion
At these elevated temperatures, atoms become mobile. They migrate across the contact points between adjacent particles, gradually forming solid bridges or "necks."
### Gradual Densification
Over time, this diffusion process causes the necks to grow and the voids (pores) between particles to shrink and close. This slowly transforms the loose powder into a dense, solid component.
The Mechanism of Liquid State Sintering
Liquid state sintering leverages a small amount of liquid to overcome the slow pace of solid-state diffusion, making the process faster and more efficient.
### Introducing a Liquid Phase
This process involves mixing the primary powder with a small amount of an additive that has a lower melting point. When heated, the additive melts and forms a liquid that coats the solid primary particles.
### Rapid Particle Rearrangement
The surface tension of this liquid creates strong capillary forces. These forces pull the solid particles together, rapidly rearranging them into a more tightly packed configuration and significantly reducing porosity in the initial stage.
### Dissolution and Re-precipitation
The liquid acts as a solvent, dissolving some material from the solid particles. This dissolved material then re-precipitates in the neck regions between particles, effectively filling the remaining voids and driving the component toward full density.
Understanding the Key Trade-offs
Choosing the correct sintering method requires understanding the direct consequences of using a liquid phase versus relying solely on solid-state diffusion.
### Sintering Temperature and Speed
Liquid state sintering is faster and occurs at lower temperatures. The liquid provides a high-speed pathway for material transport, accelerating densification compared to the slow atomic crawl of solid state diffusion.
### Achievable Density
Liquid state sintering generally achieves higher final densities. The liquid's ability to fill small pores and actively pull particles together is more effective at eliminating porosity than diffusion alone.
### Material Purity and Composition
This is the most critical trade-off. Solid state sintering maintains the chemical purity of the original powder. Liquid state sintering fundamentally alters the final material's composition because the solidified liquid phase remains as part of the microstructure.
Making the Right Choice for Your Goal
Your application's requirements for purity, density, and processing efficiency will determine the ideal approach.
- If your primary focus is material purity and maintaining the original composition: Solid state sintering is the only choice, as it introduces no new elements into the final part.
- If your primary focus is achieving maximum density quickly and at lower temperatures: Liquid state sintering is superior, provided the presence of the additive phase is acceptable in the final product.
- If you are working with materials that are very difficult to densify: The enhanced transport mechanisms of liquid state sintering often provide the most practical path to creating a dense, functional component.
Understanding these core mechanisms allows you to select the precise sintering strategy that balances purity, density, and efficiency for your specific material.
Summary Table:
| Feature | Solid State Sintering | Liquid State Sintering |
|---|---|---|
| Mechanism | Atomic diffusion in solid state | Liquid phase acts as transport medium |
| Temperature | High (close to melting point) | Lower |
| Speed | Slower | Faster |
| Final Density | Lower | Higher |
| Material Purity | Maintains original composition | Altered by additive phase |
| Best For | High-purity applications | Maximum density, efficiency |
Optimize Your Sintering Process with KINTEK
Choosing between liquid and solid state sintering is critical for achieving the desired material properties in your lab. Whether you prioritize ultimate purity with solid state methods or require the high-density, efficient results of liquid phase sintering, having the right equipment is essential.
KINTEK specializes in precision lab equipment and consumables designed to meet the exacting demands of advanced material processing. Our sintering furnaces and accessories provide the precise temperature control and atmosphere management needed for both techniques, ensuring reproducible results for researchers and manufacturers alike.
Let us help you enhance your sintering outcomes. Our experts can guide you to the ideal solution for your specific material goals.
Contact KINTEK today to discuss your sintering requirements and discover how our reliable lab solutions can drive your success.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- What are the disadvantages of biomass to the environment? Debunking the 'Green' Myth
- Why is high-power ultrasound utilized for MOFs in MMMs? Unlock Superior Gas Separation & Uniform Dispersion
- What are the advantages of physical vapor deposition? Achieve High-Purity, Durable Thin Films
- Can filter paper be used to separate solids from liquids? A Guide to Effective Filtration
- Why is precise process control in high-temperature calcination critical for iron-based metal oxide catalysts?
- What is the temperature of iron sintering? Achieve Optimal Sinter Quality for Your Blast Furnace
- What is the composition of an evaporator? The 3 Essential Components for Efficient Evaporation
- What is target in sputtering? The Essential Source Material for Thin-Film Deposition



















