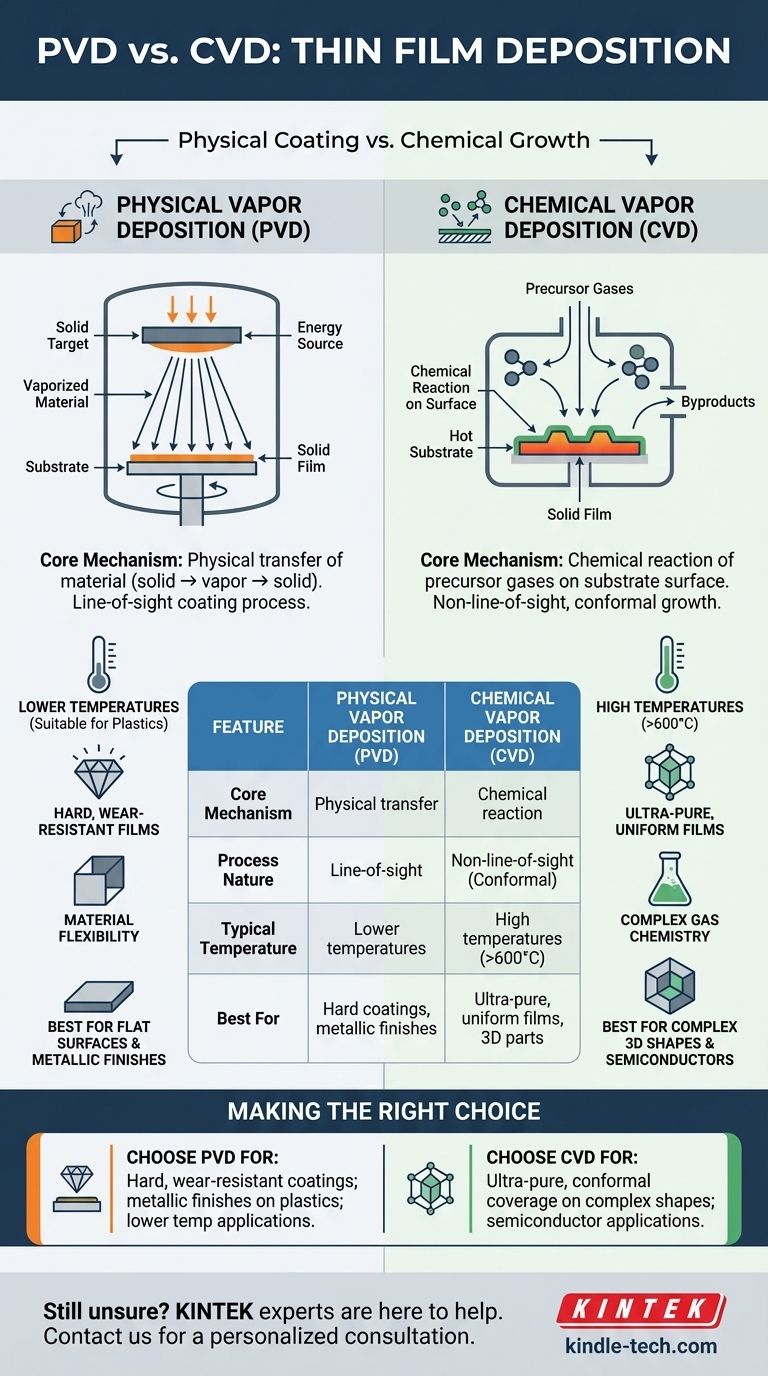
The fundamental difference between physical and chemical deposition lies in how the coating material reaches the target surface. Physical Vapor Deposition (PVD) involves physically transferring a material from a source to the substrate, essentially a change of state from solid to vapor and back to solid. In contrast, Chemical Vapor Deposition (CVD) uses precursor gases that undergo a chemical reaction directly on the substrate's surface to create a new, solid material.
While both PVD and CVD are methods for applying thin films, the core distinction is simple: PVD is a physical coating process, like spray-painting with atoms, whereas CVD is a chemical growth process, where the film is built through a reaction on the surface itself.

Deconstructing Physical Vapor Deposition (PVD)
PVD encompasses a family of processes defined by the physical movement of material. The source material is converted into a vapor, travels through a vacuum or low-pressure environment, and condenses onto the substrate as a solid film.
The Core Mechanism: A Line-of-Sight Transfer
In all PVD processes, the material being deposited starts as a solid target. Energy is applied to this target, liberating atoms or molecules that travel in a straight line until they strike a surface and stick.
This line-of-sight nature means PVD is excellent for coating flat surfaces or objects that can be easily rotated to expose all sides to the source.
Key PVD Techniques
Two primary methods are used to create the vapor:
- Evaporation: The source material is heated in a vacuum until it boils, creating a vapor that rises and coats the substrate. This is a relatively simple and gentle process.
- Sputtering: A high-energy plasma is used to bombard the source material (the "target"). This energetic collision physically knocks atoms off the target, which then travel and deposit onto the substrate.
Understanding Chemical Vapor Deposition (CVD)
CVD is fundamentally a chemical process. Instead of physically moving the final film material, it transports the chemical building blocks (precursors) to the substrate and initiates a reaction to build the film in place.
The Core Mechanism: A Surface-Based Reaction
In a typical CVD process, one or more volatile precursor gases are introduced into a reaction chamber. The substrate is heated to a specific, often high, temperature.
When the precursor gases come into contact with the hot substrate, they decompose and react with each other and the surface, forming the desired solid film. Excess gas and byproducts are then pumped out.
The Importance of Conformal Coating
Because the deposition is driven by a gas that can flow into every nook and cranny, CVD is not a line-of-sight process. It excels at producing highly conformal coatings, meaning it can deposit a film of uniform thickness over complex, three-dimensional shapes.
Understanding the Trade-offs
Choosing between PVD and CVD requires understanding their distinct advantages and limitations, which stem directly from their underlying mechanisms.
Temperature and Substrate Compatibility
CVD typically requires very high temperatures (often >600°C) to drive the necessary chemical reactions. This limits its use to substrates that can withstand that heat without melting or deforming.
PVD processes generally operate at much lower temperatures, making them suitable for a wider variety of materials, including plastics and heat-sensitive alloys.
Film Adhesion and Properties
CVD films are chemically grown on the surface, often resulting in excellent adhesion and high purity. The high temperatures can also produce a desirable crystalline structure.
PVD films, particularly from sputtering, are deposited with high kinetic energy, which creates very dense, hard, and wear-resistant coatings.
Process Complexity and Materials
CVD relies on finding suitable precursor gases that will react as intended, which can be a complex chemical challenge. The process can also involve toxic and corrosive gases.
PVD is more straightforward in principle; if you can make a target out of the material, you can likely deposit it. This offers greater flexibility for depositing alloys and composite materials.
Making the Right Choice for Your Goal
The decision to use PVD or CVD is dictated entirely by the desired outcome for the final product.
- If your primary focus is a hard, wear-resistant coating on a metal tool or a metallic finish on plastic: PVD is the clear and cost-effective choice due to its lower processing temperatures and the excellent mechanical properties of its films.
- If your primary focus is an ultra-pure, highly uniform crystalline film for semiconductors or optics: CVD is the superior method because the chemical reaction process delivers exceptional purity and conformal coverage.
- If your primary focus is coating a complex internal surface or a 3D part uniformly: CVD's non-line-of-sight nature makes it the only viable option.
Understanding whether your application requires a physical coating or a chemically grown film is the key to selecting the right tool for your engineering challenge.
Summary Table:
| Feature | Physical Vapor Deposition (PVD) | Chemical Vapor Deposition (CVD) |
|---|---|---|
| Core Mechanism | Physical transfer of material (solid → vapor → solid) | Chemical reaction of precursor gases on the substrate surface |
| Process Nature | Line-of-sight coating | Non-line-of-sight, conformal growth |
| Typical Temperature | Lower temperatures (suitable for plastics) | High temperatures (>600°C) |
| Best For | Hard, wear-resistant coatings; metallic finishes | Ultra-pure, uniform films; complex 3D shapes |
| Key Advantage | Excellent for flat surfaces; wide material flexibility | Exceptional step coverage and film purity |
Still unsure whether PVD or CVD is right for your specific application? The experts at KINTEK are here to help.
KINTEK specializes in providing advanced lab equipment and consumables for all your thin-film deposition needs. We can help you select the perfect technology to achieve the film properties, adhesion, and coverage your project requires.
Contact our specialists today for a personalized consultation and let us help you optimize your deposition process.
Get in touch with our team now!
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings
- What are the steps of the CVD process? A Guide to Precision Thin Film Deposition



















