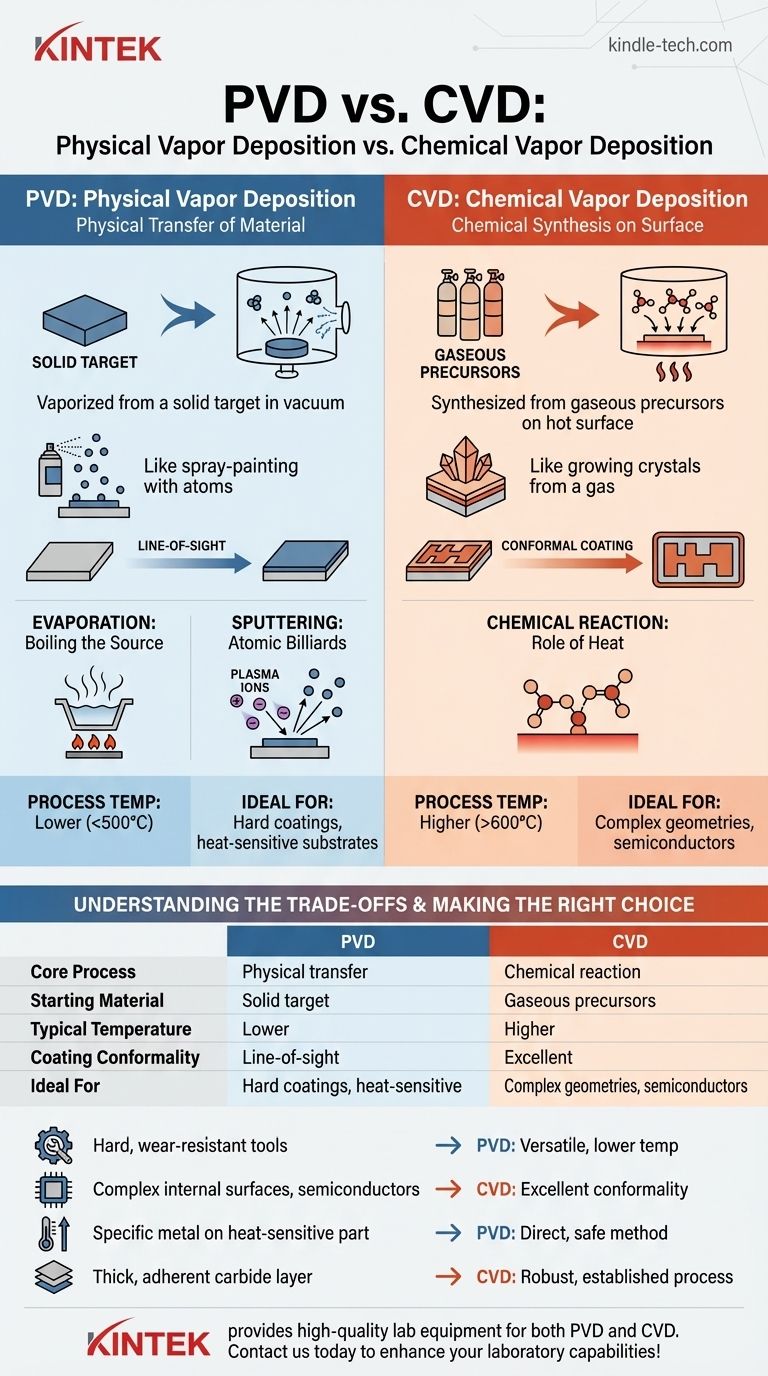
At its core, the primary difference between Physical Vapor Deposition (PVD) and Chemical Vapor Deposition (CVD) lies in how the coating material arrives at the substrate. PVD is a physical process, where a solid or liquid source material is vaporized and physically transported to the part in a vacuum. In contrast, CVD is a chemical process where gaseous molecules (precursors) react on the substrate's surface to form a new, solid film.
The simplest way to understand the distinction is through an analogy. PVD is like spray-painting with atoms, physically transferring material from a solid target onto a surface. CVD is like growing crystals from a gas, using a chemical reaction to create an entirely new solid layer on that surface.

The Mechanics of Physical Vapor Deposition (PVD)
Physical Vapor Deposition encompasses a set of vacuum deposition methods that use physical mechanisms to produce a thin film. The process involves transferring atoms or molecules directly from a source to a substrate.
The Core Principle: A Physical Transfer
In all PVD processes, a solid source material, known as the target, is placed in a vacuum chamber. Energy is applied to this target to generate a vapor of its constituent atoms or molecules.
This vapor then travels through the vacuum and condenses on the cooler substrate (the object being coated), forming a thin, solid film. It is a direct, line-of-sight process.
Evaporation: Boiling the Source Material
One major category of PVD is evaporation. In this method, the target material is heated in a vacuum until it boils, releasing a vapor. This is similar to how water boils to create steam, except it is done with metals or other compounds at much higher temperatures.
Sputtering: A Game of Atomic Billiards
The other major PVD category is sputtering. This process does not rely on melting the target. Instead, the chamber is filled with an inert gas, like Argon, which is ionized to create a plasma.
These high-energy ions are accelerated into the target, striking it with enough force to physically knock atoms loose, a process of momentum transfer. These ejected atoms then travel to the substrate and deposit as a film.
The Mechanics of Chemical Vapor Deposition (CVD)
Chemical Vapor Deposition builds films through a fundamentally different mechanism. It does not transfer existing material but rather synthesizes a new material directly on the part's surface.
The Core Principle: Building from Gaseous Precursors
In CVD, the process begins with one or more volatile gaseous chemicals, known as precursors. These gases are introduced into a reaction chamber containing the substrate.
The Role of Heat and Chemical Reaction
The substrate is typically heated to a high temperature. This thermal energy causes the precursor gases to react or decompose upon contacting the hot surface.
This chemical reaction forms a stable, solid material that deposits onto the substrate as a thin film. Gaseous byproducts of the reaction are then exhausted from the chamber.
Conformality: Coating Complex Shapes
A key advantage of CVD is its ability to produce highly conformal coatings. Because the precursor gases can flow around and into complex geometries before reacting, CVD can uniformly coat intricate shapes, channels, and even internal surfaces.
Understanding the Trade-offs
Choosing between PVD and CVD requires understanding their inherent limitations and the properties of the films they produce. The "better" process is entirely dependent on the application.
Starting Material: Solid vs. Gas
PVD can deposit almost any material that can be made into a solid target, including pure metals, alloys, and certain ceramic compounds.
CVD is limited to materials for which suitable, stable, and often toxic or corrosive precursor gases exist. The chemistry must be correct.
Process Temperature: Impact on Substrate
CVD processes typically operate at very high temperatures (often >600°C) to drive the necessary chemical reactions. This can damage or warp heat-sensitive substrates, such as hardened steels or aluminum alloys.
PVD can be performed at much lower temperatures (often <500°C), making it suitable for a wider range of substrate materials.
Coating Geometry: Line-of-Sight vs. Conformal
The line-of-sight nature of PVD means it struggles to coat complex internal features or the backside of a part without complex fixturing and rotation.
CVD's gas-phase transport gives it excellent conformality, making it the ideal choice for uniformly coating non-flat or intricate components.
Film Properties and Adhesion
PVD films are often very dense and can be deposited with high compressive stress, which is beneficial for the wear resistance of cutting tools.
CVD coatings typically have excellent adhesion due to the chemical bond formed with the substrate, but they may have different stress profiles and microstructures compared to PVD films.
Making the Right Choice for Your Goal
Your choice must be driven by your material, the geometry of your part, and the properties you need from the final coating.
- If your primary focus is hard, wear-resistant coatings on tools with simple geometries: PVD is often the more versatile and lower-temperature choice.
- If your primary focus is coating complex internal surfaces or creating ultra-pure semiconductor layers: CVD is superior due to its excellent conformality and the precision of chemical control.
- If your primary focus is depositing a specific metal or complex alloy on a heat-sensitive part: PVD is the most direct and safest method.
- If your primary focus is creating a thick, highly adherent carbide or nitride layer on a temperature-tolerant substrate: CVD is a robust and well-established industrial process.
Ultimately, understanding whether your application demands a physical transfer or a chemical synthesis is the key to selecting the correct deposition technology.
Summary Table:
| Feature | Physical Vapor Deposition (PVD) | Chemical Vapor Deposition (CVD) |
|---|---|---|
| Core Process | Physical transfer (evaporation/sputtering) | Chemical reaction on substrate surface |
| Starting Material | Solid target | Gaseous precursors |
| Typical Temperature | Lower (<500°C) | Higher (>600°C) |
| Coating Conformality | Line-of-sight (less conformal) | Excellent (highly conformal) |
| Ideal For | Hard coatings, heat-sensitive substrates | Complex geometries, semiconductors |
Struggling to choose the right deposition technology for your lab's thin-film applications? KINTEK specializes in providing high-quality lab equipment and consumables for both PVD and CVD processes. Our experts can help you select the ideal system to achieve precise, uniform coatings for your specific materials and part geometries. Contact us today via our [#ContactForm] to discuss your project requirements and discover how KINTEK can enhance your laboratory's capabilities and efficiency.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What machine is used to make lab-grown diamonds? Discover the HPHT & CVD Technologies
- What is the specific function of the metal filament in HF-CVD? Key Roles in Diamond Growth
- What is the hot filament chemical vapour deposition of diamond? A Guide to Synthetic Diamond Coating
- How does a Hot Filament Chemical Vapor Deposition (HFCVD) reactor function? Expert Guide to Diamond Film Fabrication
- What are the advantages of using HFCVD for BDD electrodes? Scaling Industrial Diamond Production Efficiently



















