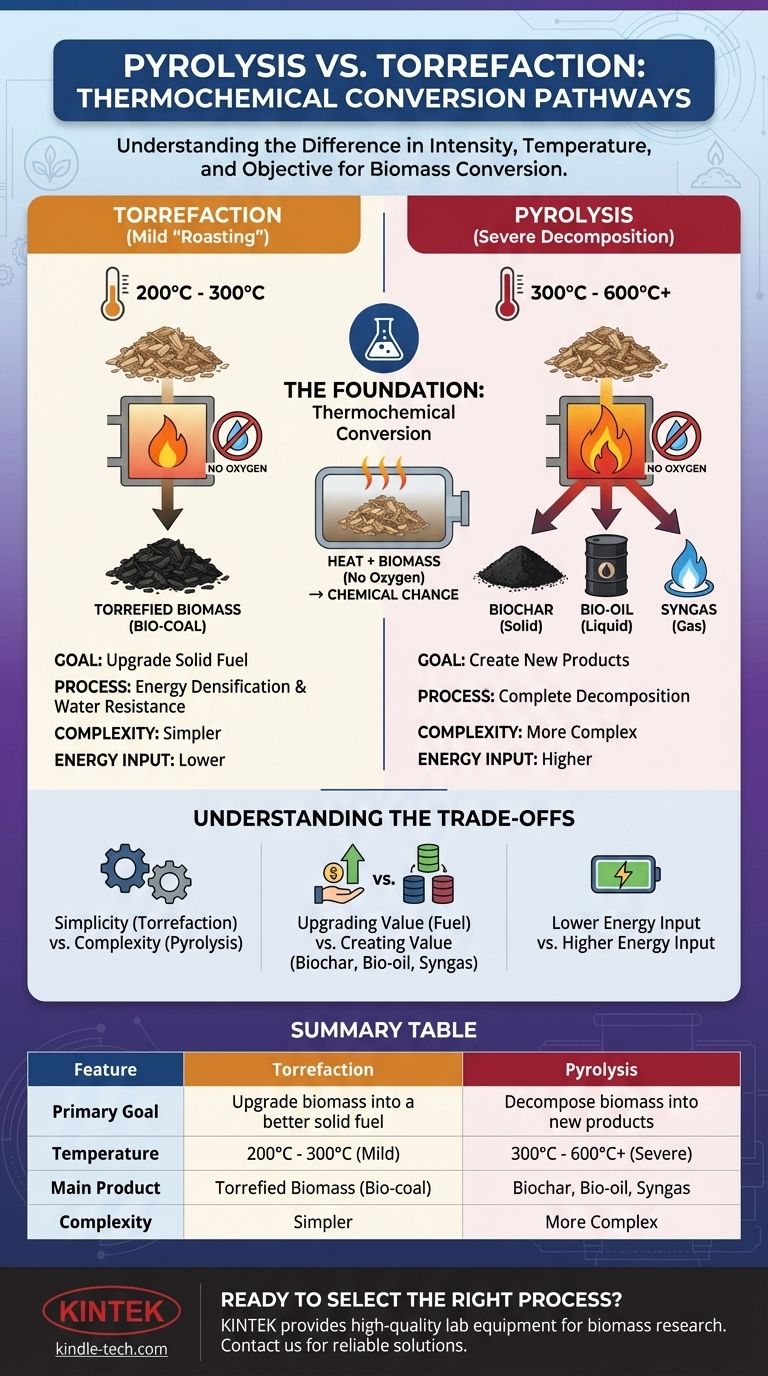
In short, pyrolysis and torrefaction are both processes that use heat to break down biomass in the absence of oxygen. The fundamental difference lies in their intensity and objective: torrefaction is a mild pre-treatment designed to upgrade biomass into a better solid fuel, while pyrolysis is a more severe process designed to completely decompose biomass into a mix of solid biochar, liquid bio-oil, and combustible gases.
The core distinction is one of purpose and temperature. Torrefaction is a low-temperature "roasting" to improve an existing fuel, whereas pyrolysis is a high-temperature decomposition to create entirely new products.

The Foundation: Thermochemical Conversion
To understand these processes, we must first grasp the principle they share. Both are forms of thermochemical conversion, which involves using heat to chemically change a material.
The Critical Role of Heat and Oxygen
In both torrefaction and pyrolysis, biomass (like wood, agricultural waste, or manure) is heated in an environment with very little to no oxygen. This is the key.
Without oxygen, the material cannot combust or burn. Instead of releasing energy as fire, the heat breaks down the complex chemical bonds within the biomass itself.
Torrefaction: Upgrading Biomass into Bio-Coal
Torrefaction is best understood as a mild form of pyrolysis, essentially a "roasting" process for biomass. Its primary goal is not to create new products but to improve the properties of the original biomass as a solid fuel.
The Goal: Energy Densification and Water Resistance
Raw biomass is often bulky, wet, and prone to biological degradation. Torrefaction addresses these issues by making the material more energy-dense, brittle (easier to grind), and hydrophobic (resistant to absorbing water). This makes it much easier to transport, store, and use in existing power plants, often alongside coal.
Key Process Parameters
The defining characteristic of torrefaction is its low operating temperature, typically between 200°C and 300°C. At this temperature, the process drives off water and breaks down the less stable organic compounds (hemicellulose) in the biomass.
Primary Output: Torrefied Biomass
The main product is a dry, blackened, solid material often called torrefied biomass or bio-coal. While some combustible gases are released and can be used to power the process, the solid product is the primary focus. It retains most of its original energy content but in a much more compact and stable form.
Pyrolysis: Decomposing Biomass into New Products
Pyrolysis operates at higher temperatures to achieve a much more radical transformation. Its goal is to completely decompose the biomass into a valuable slate of new solid, liquid, and gaseous products.
The Goal: Creating Biochar, Bio-oil, and Syngas
Unlike torrefaction, pyrolysis is not about improving an existing fuel. It is a conversion technology designed to break down the feedstock into fundamental components: a solid carbon char, a liquid oil, and a mixture of flammable gases.
Key Process Parameters
Pyrolysis takes place at higher temperatures, typically from 300°C up to 600°C or more, in the complete absence of oxygen. The speed of the heating process (fast or slow pyrolysis) dramatically changes the ratio of the final products.
Primary Outputs: A Mix of Solids, Liquids, and Gases
Pyrolysis yields three distinct products:
- Biochar (Solid): A stable, carbon-rich charcoal used for soil amendment, carbon sequestration, or filtration. Slow pyrolysis maximizes biochar yield.
- Bio-oil (Liquid): A dark, viscous liquid that can be refined into transportation fuels or used as a source for specialty chemicals. Fast pyrolysis maximizes bio-oil yield.
- Syngas (Gas): A mixture of combustible gases (like hydrogen, carbon monoxide, and methane) that can be used to generate heat and power, often to sustain the pyrolysis process itself.
Understanding the Trade-offs
Choosing between these two technologies requires understanding their inherent advantages and disadvantages, which are directly linked to their different operating conditions and goals.
Simplicity vs. Complexity
Torrefaction is a relatively simpler and more robust process. The lower temperatures and focus on a single solid output make the equipment and operations less demanding.
Pyrolysis is significantly more complex. Managing higher temperatures and handling three different product streams (solid, liquid, and gas) requires more sophisticated engineering and control systems.
Upgrading vs. Creating Value
Torrefaction adds value by upgrading a low-quality feedstock into a high-quality solid fuel, reducing logistical costs and improving handling.
Pyrolysis creates value by converting a low-value feedstock into multiple, distinct, and potentially higher-value products like biochar for agriculture or bio-oil for the chemical industry.
Energy Input
Because it operates at lower temperatures, torrefaction requires less energy input to process the same amount of biomass. Pyrolysis is a more energy-intensive process due to the need to achieve and maintain much higher temperatures for complete decomposition.
Making the Right Choice for Your Goal
Your choice depends entirely on your desired outcome. The question is not which process is "better," but which is the right tool for your specific objective.
- If your primary focus is improving the handling, storage, and combustion properties of raw biomass for co-firing in power plants: Torrefaction is the ideal and most direct solution.
- If your primary focus is producing a stable, carbon-rich soil amendment to improve agricultural land and sequester carbon: Slow pyrolysis is the correct process to maximize biochar yield.
- If your primary focus is creating a liquid bio-fuel or a feedstock for renewable chemicals: Fast pyrolysis is the technology designed to maximize the liquid bio-oil fraction.
By understanding this fundamental distinction in temperature and intent, you can confidently select the right thermochemical pathway to meet your material and energy objectives.
Summary Table:
| Feature | Torrefaction | Pyrolysis |
|---|---|---|
| Primary Goal | Upgrade biomass into a better solid fuel | Decompose biomass into new products (biochar, bio-oil, syngas) |
| Temperature Range | 200°C - 300°C (Mild) | 300°C - 600°C+ (Severe) |
| Main Product | Torrefied Biomass (Bio-coal) | Biochar (Slow), Bio-oil (Fast), and Syngas |
| Process Complexity | Simpler | More Complex |
Ready to select the right thermochemical process for your biomass conversion needs?
KINTEK specializes in providing high-quality lab equipment and consumables for advanced biomass research and development. Whether you are exploring torrefaction for fuel upgrading or pyrolysis for biochar and bio-oil production, our reliable solutions can support your work.
Contact us today using the form below to discuss how our expertise can help you achieve your material and energy objectives.
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- How do tube furnaces or muffle furnaces ensure stoichiometric accuracy during synthesis? Mastering Li4GeO4 & Li4VO4
- How are tube furnaces classified based on the orientation of the tube? Choose the Right Design for Your Process
- How are composites processed using sintering? Engineered Material Solutions Through Advanced Thermal Bonding
- Why is a high-temperature furnace with multi-probe testing used for ABO3 perovskite? Get Precise Conductivity Data



















