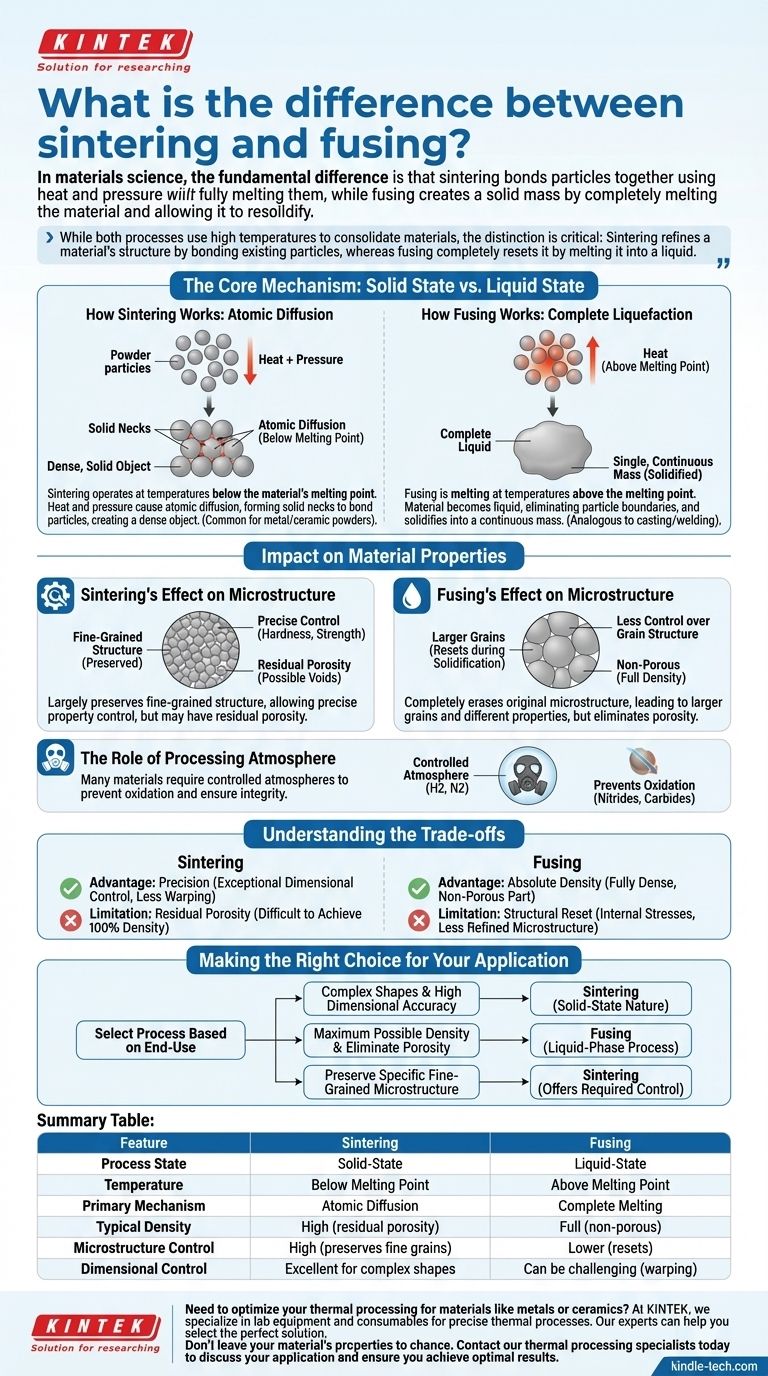
In materials science, the fundamental difference is that sintering bonds particles together using heat and pressure without fully melting them, while fusing creates a solid mass by completely melting the material and allowing it to resolidify. Sintering is a solid-state process driven by atomic diffusion, whereas fusing is a liquid-state process involving a phase change.
While both processes use high temperatures to consolidate materials, the distinction is critical: Sintering refines a material's structure by bonding existing particles, whereas fusing completely resets it by melting it into a liquid.

The Core Mechanism: Solid State vs. Liquid State
The most important distinction lies in what happens to the material at the atomic level. One process carefully rearranges the existing structure, while the other completely liquefies it.
How Sintering Works: Atomic Diffusion
Sintering operates at temperatures below the material's melting point.
Heat gives the atoms in individual powder particles enough energy to move. Under pressure, these mobile atoms diffuse across the boundaries between particles, forming solid "necks" that bridge the gaps.
As this process continues, the particles bond, the gaps (pores) between them shrink, and the component becomes a dense, solid object. This is common for producing parts from metal and ceramic powders.
How Fusing Works: Complete Liquefaction
Fusing is a more straightforward concept: melting.
The material is heated above its melting point until it becomes a complete liquid. In this liquid state, the original particle boundaries are entirely eliminated.
Upon cooling, the liquid solidifies into a single, continuous mass. This is analogous to casting or welding, where the goal is to create a fully dense, monolithic structure.
Impact on Material Properties
The choice between these two methods has significant consequences for the final component's internal structure and performance.
Sintering's Effect on Microstructure
Because sintering doesn't involve bulk melting, it can largely preserve the fine-grained microstructure of the starting powders.
This gives engineers precise control over material properties like hardness and strength. However, it can be challenging to eliminate all porosity, which may remain as tiny voids in the final part.
Fusing's Effect on Microstructure
Fusing completely erases the original microstructure. The new structure is formed during solidification, which can lead to larger grains and a different set of mechanical properties.
While this process naturally eliminates the porosity found in powder-based methods, it offers less control over the final grain structure.
The Role of Processing Atmosphere
For many advanced materials, the environment during heating is critical.
Nitrides, carbides, and many metals require sintering under a controlled atmosphere, such as hydrogen or nitrogen gas. This prevents oxidation and other chemical reactions that would compromise the integrity of the final part.
Understanding the Trade-offs
Neither method is universally superior. The correct choice depends on balancing the need for density against the need for structural control.
The Advantage of Sintering: Precision
Sintering provides exceptional dimensional control. Since the material never becomes a free-flowing liquid, parts are less likely to warp or slump, making it ideal for manufacturing complex net-shape components.
The Limitation of Sintering: Residual Porosity
Achieving 100% theoretical density through sintering alone can be difficult and expensive. The small amount of remaining porosity can sometimes be a limiting factor for high-stress applications.
The Advantage of Fusing: Absolute Density
The primary benefit of fusing is the straightforward creation of a fully dense, non-porous part. The liquid material naturally fills all voids, ensuring a solid final product.
The Limitation of Fusing: Structural Reset
The complete melting and resolidification can introduce internal stresses and a less refined microstructure. This lack of control can be a significant disadvantage for high-performance components.
Making the Right Choice for Your Application
Selecting the right thermal process is determined by the end-use requirements of your component.
- If your primary focus is creating complex shapes with high dimensional accuracy: Sintering is the superior method due to its solid-state nature.
- If your primary focus is achieving the maximum possible density and eliminating all porosity: Fusing or a similar liquid-phase process is the necessary approach.
- If your primary focus is preserving a specific, fine-grained microstructure for optimal mechanical properties: Sintering offers the control required to achieve this.
Ultimately, understanding whether your material needs to remain solid or become liquid during processing is the key to mastering its final form and function.
Summary Table:
| Feature | Sintering | Fusing |
|---|---|---|
| Process State | Solid-State | Liquid-State |
| Temperature | Below Melting Point | Above Melting Point |
| Primary Mechanism | Atomic Diffusion | Complete Melting |
| Typical Density | High (may have residual porosity) | Full (non-porous) |
| Microstructure Control | High (preserves fine grains) | Lower (resets during solidification) |
| Dimensional Control | Excellent for complex shapes | Can be challenging due to warping |
Need to optimize your thermal processing for materials like metals or ceramics?
Choosing the right method—sintering or fusing—is critical to achieving the desired density, microstructure, and performance in your final component. The wrong choice can lead to costly failures or subpar results.
At KINTEK, we specialize in the lab equipment and consumables that power these precise thermal processes. Whether you require a controlled atmosphere furnace for sintering sensitive materials or a high-temperature system for fusing applications, our experts can help you select the perfect solution for your laboratory's specific needs.
Don't leave your material's properties to chance. Contact our thermal processing specialists today to discuss your application and ensure you achieve optimal results.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Which type of metals can be melted using tilting furnace? Your Guide to Ferrous, Non-Ferrous & Precious Metals
- Why is a high-precision industrial electric furnace required for metal normalizing? Unlock Superior Grain Refinement
- How does high-temperature heating equipment simulate the service environment of ceramics? Expert Testing Strategies
- Why is a constant temperature stirring reactor necessary for castor oil transesterification? Optimize Biodiesel Yields
- What are the hazards of quenching? Avoid Material Failure and Personnel Injury
- What is a belt type furnace? Achieve High-Volume Heat Treatment for Small Parts
- Why is it necessary to strictly control IZO sintering cooling and pressure? Prevent Thermal Shock & Ceramic Failure
- What are the technical advantages of using an RMI furnace? Achieve High-Density Ceramics with Precision



















