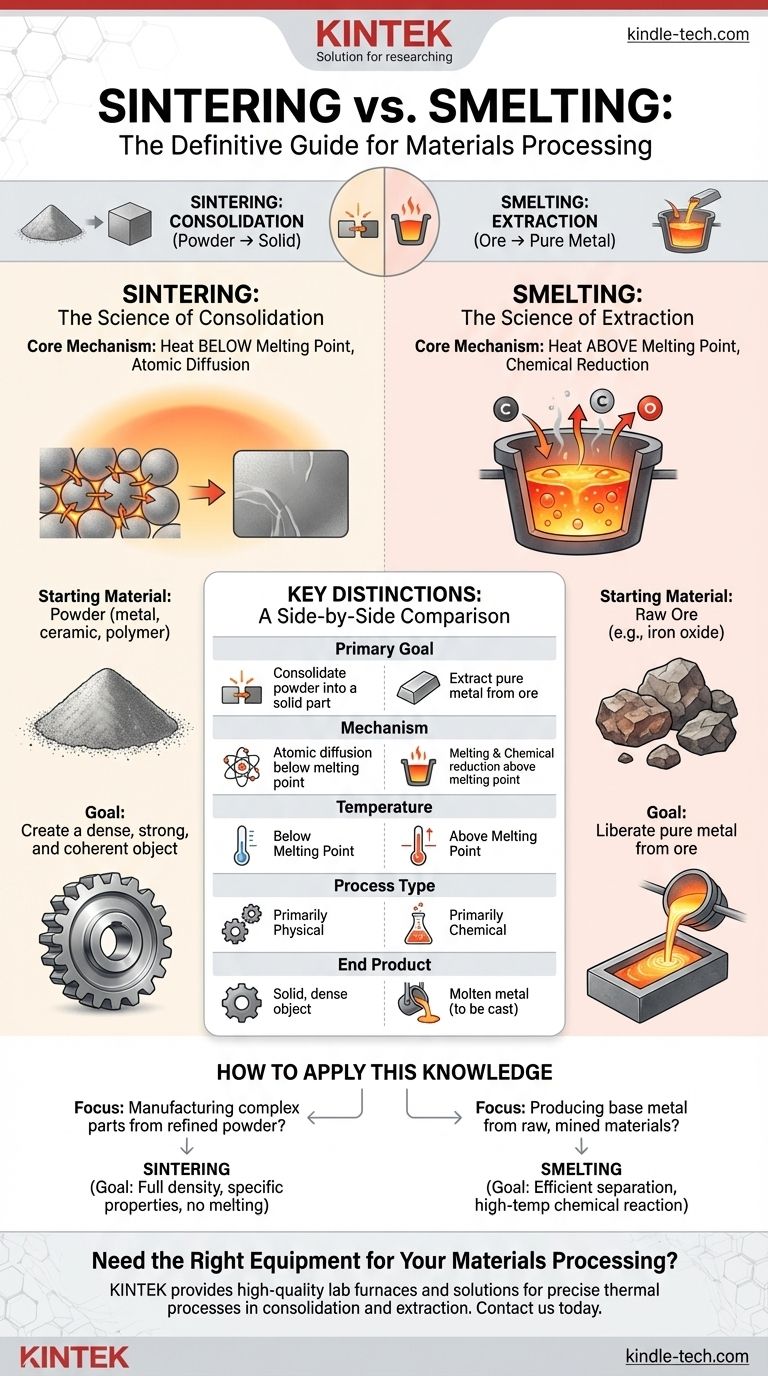
At a fundamental level, the difference between sintering and smelting comes down to their purpose and mechanism. Sintering is a process that fuses powdered materials into a solid mass using heat below the melting point. In contrast, smelting uses heat above the melting point along with chemical agents to extract a pure metal from its ore.
Sintering is a process of consolidation, turning a powder into a dense solid object. Smelting is a process of extraction, chemically separating a pure metal from its natural ore. The choice depends entirely on whether your starting material is a powder you want to solidify or an ore you need to purify.

What is Sintering? The Science of Consolidation
Sintering is a thermal treatment for compacting and forming a solid material from a powder without melting it to the point of liquefaction.
The Core Mechanism: Heat, Not Melting
The driving force of sintering is atomic diffusion. When a powdered mass is heated, the atoms at the contact points between particles become more mobile. They migrate across the boundaries, effectively fusing the particles together and gradually reducing the empty space, or porosity, between them.
This entire process occurs at temperatures below the material's melting point. Think of how tightly packed snow under pressure can slowly turn to solid ice, even below freezing—it's a change in structure, not a change of state from solid to liquid and back.
The Starting Material: Powder
Sintering begins with a powder of a specific material, such as a metal alloy, a ceramic, or a polymer. This powder is often first compressed into a desired shape, creating a fragile object sometimes called a "green part."
The Goal: Creating a Dense, Solid Part
The ultimate goal of sintering is to create a dense, strong, and coherent object from the initial powder. It is a cornerstone of powder metallurgy, ceramic manufacturing, and certain types of additive manufacturing (3D printing).
What is Smelting? The Science of Extraction
Smelting is a far more aggressive process used in extractive metallurgy to produce a base metal from its natural ore.
The Core Mechanism: Melting and Chemical Reduction
Smelting involves heating the ore to temperatures well above the metal's melting point. Critically, this process also involves a chemical reduction reaction.
A reducing agent, such as carbon (in the form of coke), is added to the furnace with the ore. At high temperatures, the carbon chemically strips the oxygen atoms away from the metal oxide, leaving behind a molten, purified metal.
The Starting Material: Ore
The input for smelting is a raw ore, which is a chemical compound mined from the earth. A common example is iron ore (iron oxide), which is not metallic iron but a compound containing iron.
The Goal: Liberating Pure Metal
The goal of smelting is to separate the desired metal from the other elements in the ore. The output is a molten metal and a waste by-product called slag, which contains the impurities.
Understanding the Key Distinctions
While both are high-temperature processes, their fundamental differences dictate their application.
Process Goal: Consolidation vs. Extraction
Sintering consolidates a powder into a solid shape. Smelting extracts a pure metal from a chemical compound (ore). You sinter a refined material; you smelt a raw material.
Temperature: Below vs. Above Melting Point
Sintering works by making atoms mobile below the melting point. Smelting relies on fully melting the material to facilitate the chemical separation.
Transformation Type: Physical vs. Chemical
Sintering is primarily a physical process of particles fusing together. Smelting is fundamentally a chemical process of reduction, where the ore is chemically transformed into a pure metal.
Material End State: Solid vs. Molten
Sintering typically results in a solid, finished part. Smelting results in a molten metal that must then be cast into a shape, like an ingot.
How to Apply This Knowledge
Choosing between these processes is not a matter of preference but of necessity based on your starting material and end goal.
- If your primary focus is manufacturing complex parts from a refined powder (metal or ceramic): You are concerned with sintering. Your goal is to achieve full density and specific mechanical properties without melting the material.
- If your primary focus is producing base metal from raw, mined materials: You are concerned with smelting. Your goal is to efficiently separate the metal from its ore through a high-temperature chemical reaction.
Understanding this distinction between consolidation and extraction is fundamental to mastering materials processing.
Summary Table:
| Feature | Sintering | Smelting |
|---|---|---|
| Primary Goal | Consolidate powder into a solid part | Extract pure metal from ore |
| Mechanism | Atomic diffusion below melting point | Melting & chemical reduction above melting point |
| Starting Material | Powder (metal, ceramic, polymer) | Ore (e.g., iron oxide) |
| End Product | Solid, dense object | Molten metal (to be cast) |
| Process Type | Primarily physical | Primarily chemical |
Need the Right Equipment for Your Materials Processing?
Whether your lab focuses on sintering powdered materials for advanced components or requires robust solutions for metallurgical testing, KINTEK has the expertise and equipment to support your work. We specialize in high-quality lab furnaces, consumables, and accessories tailored for precise thermal processes.
Contact us today to discuss how our solutions can enhance your efficiency, accuracy, and results in consolidation or extraction applications.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What is vacuum brazed? The Ultimate Guide to High-Purity Metal Joining
- What are some applications of brazing? Join Dissimilar Metals with Strong, Leak-Proof Bonds
- What are the factors that affect the strength of a brazed joint? Master the 4 Keys to a Perfect Bond
- What is the basic of brazing? A Guide to Strong, Low-Heat Metal Joining
- What are vacuum furnaces used for? Unlock Ultimate Material Purity and Performance


















