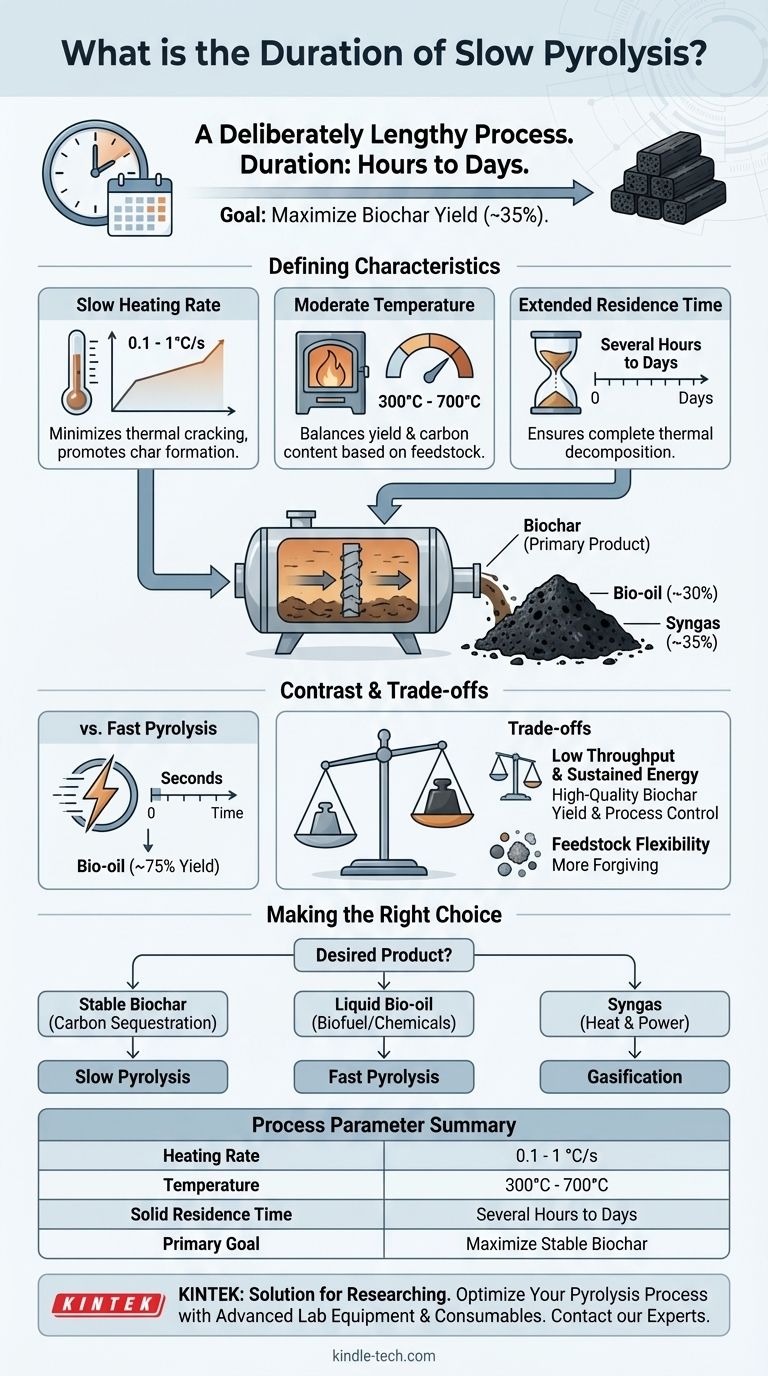
In short, slow pyrolysis is a deliberately lengthy process. Its duration can range from several hours to even days, depending on the specific feedstock, reactor scale, and desired product characteristics. This extended timeframe is a direct result of its very low heating rate, which is the defining feature of the process.
The long duration of slow pyrolysis is not an inefficiency but a fundamental requirement. It is intentionally slow to maximize the conversion of biomass into biochar, the primary and most valuable product of this specific thermal process.

The Defining Characteristics of Slow Pyrolysis
To understand the duration, we must first understand the core parameters that define slow pyrolysis. The process is a careful balance of temperature, heating rate, and time, all optimized for one specific outcome.
The Critical Role of a Slow Heating Rate
The most important factor is the slow heating rate, typically between 0.1 and 1°C per second.
This gradual increase in temperature is crucial. It minimizes the rapid thermal cracking that produces liquids and gases, instead promoting secondary reactions that polymerize and re-condense molecules into a stable, solid carbon structure—the biochar.
Operating Temperature Range
Slow pyrolysis is generally conducted at moderate temperatures, often between 300°C and 700°C.
The specific temperature is chosen based on the feedstock and the desired properties of the final biochar. Lower temperatures tend to produce higher yields, while higher temperatures increase the carbon content and stability of the char.
Extended Residence Time
The residence time—the period the material is held at the peak temperature—is very long.
This can be anywhere from 5 to 30 minutes for vapors and several hours or even days for the solid biomass. This long duration ensures the thermal decomposition is complete, maximizing the conversion to biochar.
Why Such a Long Duration? The Goal is Biochar
The relationship between the process parameters and the final product is the key to understanding slow pyrolysis. Unlike other methods, its goal is not energy or liquid fuel, but a solid material.
Maximizing Solid Product Yield
The primary goal is to produce the maximum possible amount of biochar. Slow heating and long residence times create the ideal conditions for its formation, typically yielding around 35% biochar by weight, with the rest being bio-oil (30%) and syngas (35%).
Contrast with Fast Pyrolysis
This stands in stark contrast to fast pyrolysis, which uses extremely high heating rates and a residence time of only a few seconds. Fast pyrolysis is designed to maximize the liquid bio-oil yield (up to 75%) by rapidly vaporizing the biomass before it can form char.
Understanding the Trade-offs
Choosing a thermal process involves clear trade-offs between speed, product yield, and operational complexity. Slow pyrolysis is no exception.
Throughput vs. Biochar Yield
The most significant trade-off is processing speed versus product output. Slow pyrolysis has a very low throughput, meaning it can process relatively little material in a given period. However, it provides the highest possible yield of high-quality biochar.
Energy Input and Control
Maintaining a reactor at a specific temperature for many hours requires a sustained energy input. However, the slow, steady nature of the process can make it easier to control and stabilize compared to the volatile, near-instantaneous reactions of fast pyrolysis.
Feedstock Preparation
Slow pyrolysis is generally more forgiving of larger particle sizes and variations in feedstock compared to fast pyrolysis, which requires finely ground, dry material for its rapid heat transfer to work effectively.
Making the Right Choice for Your Goal
The "best" pyrolysis method is entirely dependent on your desired end product. The duration is simply a consequence of that goal.
- If your primary focus is producing stable biochar for carbon sequestration or soil amendment: Slow pyrolysis is the correct and most effective method.
- If your primary focus is creating liquid bio-oil for use as a biofuel or chemical feedstock: Fast pyrolysis is the necessary choice due to its rapid heating and short duration.
- If your primary focus is generating syngas for heat and power: Gasification, a related process at higher temperatures, is the most efficient pathway.
Ultimately, the duration of slow pyrolysis is a deliberate and necessary feature designed to create the optimal environment for forming a stable, solid carbon product.
Summary Table:
| Process Parameter | Typical Range for Slow Pyrolysis |
|---|---|
| Heating Rate | 0.1 - 1 °C per second |
| Temperature | 300°C - 700°C |
| Solid Residence Time | Several hours to days |
| Primary Product Yield | ~35% Biochar |
| Goal | Maximize stable, solid carbon (biochar) production |
Ready to Optimize Your Pyrolysis Process?
Choosing the right thermal conversion technology is critical to achieving your specific product goals, whether it's high-quality biochar, bio-oil, or syngas. The precise control of parameters like duration, temperature, and heating rate is key.
KINTEK specializes in advanced lab equipment and consumables for pyrolysis research and development. Our reactors and analytical tools are designed to provide the reliability and control you need to accurately study processes like slow pyrolysis, optimize conditions, and scale your technology effectively.
Let us help you build a more efficient and productive laboratory. Contact our experts today to discuss how our solutions can support your biomass conversion projects.
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is a rotary retort furnace? Achieve Superior Uniformity in Continuous Heat Treatment
- What is a rotary heat type furnace? The Ultimate Guide to Uniform Heating & Mixing
- Why is a high-temperature furnace with multi-probe testing used for ABO3 perovskite? Get Precise Conductivity Data
- What is the temperature of a rotary hearth furnace? Find the Right Heat for Your Process
- How are composites processed using sintering? Engineered Material Solutions Through Advanced Thermal Bonding



















