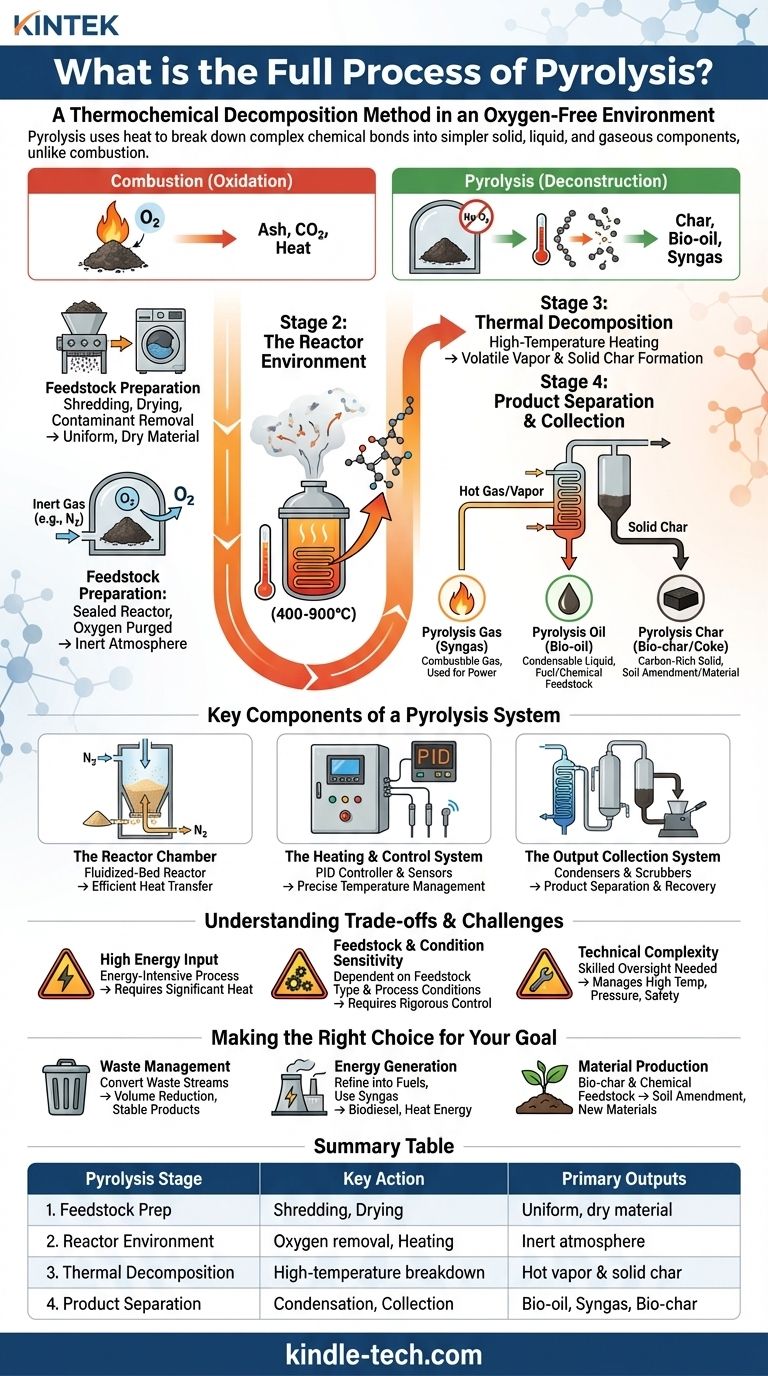
To define the full process of pyrolysis, it is a method of thermochemical decomposition where a material is subjected to extremely high temperatures in a completely oxygen-free environment. Unlike combustion (burning), which is an oxidation process, pyrolysis uses heat to break down the complex chemical bonds within a substance, deconstructing it into simpler, more valuable solid, liquid, and gaseous components.
Pyrolysis is not a process of destruction, but one of transformation. It carefully dismantles materials at a molecular level using heat, converting what is often considered waste into a predictable set of useful outputs: a solid char, a liquid oil, and a combustible gas.

The Core Principle: Deconstruction Without Oxygen
Why the Absence of Oxygen is Critical
The defining characteristic of pyrolysis is the inert (oxygen-free) atmosphere. Introducing oxygen would cause the material to combust, or burn, releasing its energy as heat and producing ash and flue gases like carbon dioxide.
By eliminating oxygen, the intense heat (typically 400-900°C) cannot burn the material. Instead, it acts as a molecular hammer, shattering long-chain molecules into smaller, more stable fragments.
A Simple Chemical Example
Consider the pyrolysis of methane (CH₄). Heat is applied to break the strong carbon-hydrogen bonds. The result is not CO₂ and water (as in combustion), but pure hydrogen gas (H₂) and solid carbon (C)—two distinct and valuable products. This same principle applies to more complex materials like biomass or plastic.
A Step-by-Step Breakdown of the Pyrolysis Process
The process can be understood as a controlled, four-stage workflow from raw material to finished product.
Stage 1: Feedstock Preparation
Before entering the reactor, the raw material—such as plastic, used tires, or woody biomass—is often prepared. This can include shredding, drying, and removing contaminants to ensure a uniform size and moisture content for efficient processing.
Stage 2: The Reactor Environment
The prepared feedstock is fed into a sealed reactor chamber. This chamber is then purged of all oxygen, typically by introducing an inert gas like nitrogen. This step is crucial for preventing unwanted combustion reactions.
Stage 3: Thermal Decomposition
Once the material is sealed in the inert environment, the heating system is activated. As the temperature rises, the feedstock undergoes thermal decomposition. Volatile compounds vaporize and exit the reactor as a hot gas and vapor stream, while the non-volatile, carbon-rich material remains as a solid.
Stage 4: Product Separation and Collection
The stream of hot gas and vapor is directed out of the reactor and into a separation and condensation system.
- Pyrolysis Gas (Syngas): The non-condensable gases are separated out. This syngas is highly combustible and is often routed back to power the plant's own heating system, making the process partially self-sustaining.
- Pyrolysis Oil (Bio-oil): The condensable vapors are cooled, causing them to liquefy into bio-oil, which is collected in tanks.
- Pyrolysis Char (Bio-char/Coke): The solid, carbon-rich material left behind in the reactor is removed after the cycle is complete.
Key Components of a Pyrolysis System
A functional pyrolysis plant is more than just a furnace; it is a precisely controlled system.
The Reactor Chamber
This is the core vessel where decomposition occurs. A common and efficient design is the fluidized-bed reactor, which contains a layer of sand. The inert gas (nitrogen) is pumped up through the sand, causing it to behave like a fluid, which ensures extremely efficient and uniform heat transfer to the feedstock.
The Heating and Control System
Modern systems use electric heating elements governed by sophisticated controls. A PID (Proportional-Integral-Derivative) controller and sensitive sensors continuously monitor and adjust the temperature, ensuring the process runs under optimal, predetermined conditions for the specific feedstock.
The Output Collection System
This includes the condensers required to turn the hot vapor stream into liquid bio-oil, as well as the piping and scrubbers for the syngas and the mechanical systems for extracting the solid bio-char.
Understanding the Trade-offs and Challenges
While powerful, pyrolysis is a technology with specific operational realities that must be managed.
High Energy Input
Reaching and maintaining temperatures of up to 900°C is an energy-intensive process. While the use of syngas can offset some of this demand, the initial energy requirement remains a significant factor in operational efficiency.
Feedstock and Condition Sensitivity
The exact composition and yield of the three outputs—gas, liquid, and solid—are highly dependent on both the type of feedstock and the precise process conditions (temperature, heating rate). Achieving a consistent product requires rigorous control over inputs and operations.
Technical Complexity
Operating a pyrolysis plant is not a simple task. It requires skilled oversight to manage the high temperatures, pressures, and control systems needed to ensure both safety and the production of high-quality outputs.
Making the Right Choice for Your Goal
The application of pyrolysis is best understood by its intended outcome.
- If your primary focus is waste management: Pyrolysis is an exceptional tool for converting problematic waste streams like plastics and tires into a smaller volume of stable, potentially valuable products.
- If your primary focus is energy generation: The bio-oil can be refined into fuels like biodiesel, and the syngas provides a direct source of heat energy, often for the plant itself.
- If your primary focus is material production: Bio-char is a valuable soil amendment and carbon sequestration tool, while bio-oil serves as a feedstock for producing other chemicals.
Pyrolysis is a versatile technology that transforms materials by deconstruction, unlocking the value held within their chemical bonds.
Summary Table:
| Pyrolysis Stage | Key Action | Primary Outputs |
|---|---|---|
| 1. Feedstock Prep | Shredding, Drying | Uniform, dry material |
| 2. Reactor Environment | Oxygen removal, Heating | Inert atmosphere |
| 3. Thermal Decomposition | High-temperature breakdown | Hot vapor & solid char |
| 4. Product Separation | Condensation, Collection | Bio-oil, Syngas, Bio-char |
Ready to transform your waste streams into valuable resources? KINTEK specializes in advanced lab equipment and consumables for pyrolysis research and development. Our reactors, control systems, and condensers are designed for precise, efficient thermal decomposition. Whether you're focused on waste management, energy generation, or material production, our solutions help you achieve consistent, high-quality outputs. Contact our experts today to discuss how we can support your laboratory's pyrolysis projects.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Lab-Scale Vacuum Induction Melting Furnace
People Also Ask
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















