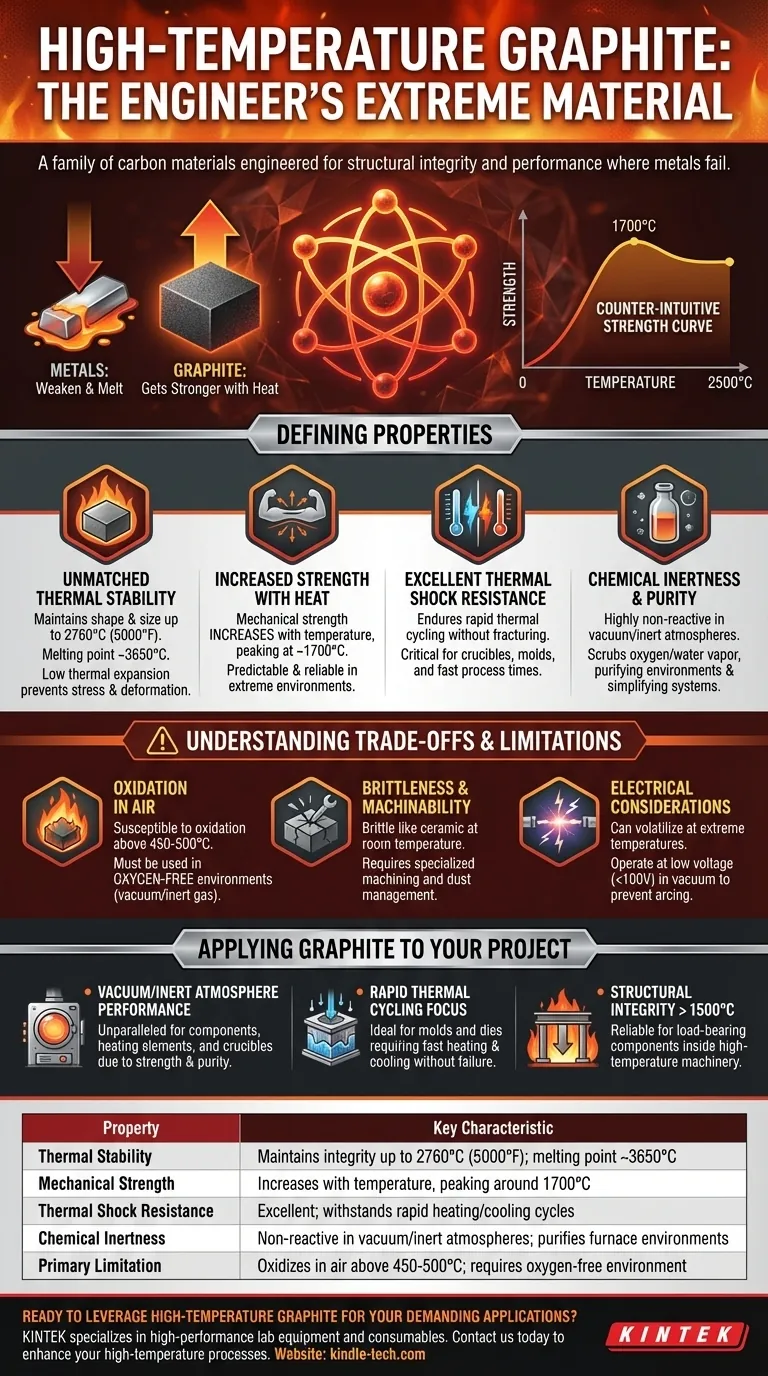
At its core, high-temperature graphite is not one specific material, but a family of crystalline carbon materials engineered to maintain structural integrity and performance at temperatures where most metals would melt or deform. Its defining characteristic is a unique atomic structure that allows it to become stronger as it gets hotter, making it an indispensable material for extreme thermal applications like vacuum furnaces and molten metal processing.
Graphite's value in high-temperature environments stems from a rare combination of properties: it resists thermal shock, has a melting point higher than most metals, and uniquely increases in mechanical strength up to approximately 2500°C.

The Defining Properties of High-Temperature Graphite
To understand why graphite is chosen for these demanding roles, we must look beyond its simple heat resistance and examine its specific thermo-mechanical behaviors.
Unmatched Thermal Stability
Graphite exhibits exceptional dimensional stability, meaning it holds its shape and size even when subjected to extreme heat. It does not melt until approximately 3650°C (6600°F) and can be used in applications up to 2760°C (5000°F).
This stability is a direct result of its low coefficient of thermal expansion. Unlike materials that expand significantly when heated, graphite changes very little, preventing stress and deformation.
The Counter-Intuitive Strength Curve
The most remarkable property of graphite is its relationship between strength and temperature. While metals weaken and creep as they are heated, graphite does the opposite.
Its mechanical strength increases with temperature, peaking around 1700°C and continuing to perform well up to 2500°C. This makes it a predictable and reliable structural material in environments where other materials would fail.
Excellent Thermal Shock Resistance
The combination of low thermal expansion and high thermal conductivity gives graphite superior resistance to thermal shock.
It can endure rapid heating and cooling cycles without cracking or fracturing. This property is critical for applications like crucibles and molds that are quickly heated and cooled, which helps to reduce overall process time.
Chemical Inertness and Purity
In a vacuum or inert atmosphere, graphite is highly non-reactive. It is often used to contain molten metals and glass because it is not easily "wetted" and does not contaminate the melt.
In vacuum furnaces, graphite components serve a dual purpose. They react with residual oxygen and water vapor, effectively "scrubbing" the atmosphere and purifying the environment. This can simplify vacuum system requirements and reduce operational costs.
Understanding the Trade-offs and Limitations
No material is perfect. While graphite's high-temperature performance is exceptional, its use is governed by critical limitations that every engineer must respect.
Oxidation in Air
Graphite's primary weakness is its susceptibility to oxidation. Its high-temperature superpowers are only unlocked in oxygen-free environments, such as a vacuum or an inert gas backfill (like argon or nitrogen).
When exposed to oxygen, graphite will begin to oxidize (burn) at temperatures as low as 450-500°C. Using it in an open-air, high-heat application will result in rapid material degradation.
Brittleness and Machinability
At room temperature, graphite is a brittle material, much like a ceramic. It must be handled and machined with care to prevent chipping or fracturing.
While it is relatively easy to machine into complex shapes, its abrasive dust requires specialized equipment and procedures to manage.
Electrical Considerations in a Vacuum
When used as a heating element in a vacuum furnace, graphite's electrical properties must be managed. It can volatilize (turn into a gas) at extremely high temperatures.
Furthermore, it is necessary to operate graphite heating elements at a low voltage (typically under 100V) to prevent electrical arcing or discharge in the vacuum environment.
How to Apply This to Your Project
Your choice to use graphite should be based on its unique strengths aligned with your specific operational environment.
- If your primary focus is performance in a vacuum or inert atmosphere: Graphite is an unparalleled choice for furnace components, heating elements, and crucibles due to its strength and chemical purity at extreme temperatures.
- If your primary focus is rapid thermal cycling: Graphite's excellent thermal shock resistance makes it ideal for molds, dies, and other parts that must be heated and cooled quickly without failing.
- If your primary focus is structural integrity above 1500°C: Graphite is one of the very few materials that gets stronger as it gets hotter, making it a reliable choice for load-bearing components inside high-temperature machinery.
By understanding its unique strengths and critical limitations, you can leverage graphite to solve thermal management challenges that few other materials can.
Summary Table:
| Property | Key Characteristic |
|---|---|
| Thermal Stability | Maintains integrity up to 2760°C (5000°F); melting point ~3650°C |
| Mechanical Strength | Increases with temperature, peaking around 1700°C |
| Thermal Shock Resistance | Excellent; withstands rapid heating/cooling cycles |
| Chemical Inertness | Non-reactive in vacuum/inert atmospheres; purifies furnace environments |
| Primary Limitation | Oxidizes in air above 450-500°C; requires oxygen-free environment |
Ready to leverage high-temperature graphite for your demanding applications?
KINTEK specializes in high-performance lab equipment and consumables, including graphite components designed for extreme environments. Whether you need custom crucibles, furnace elements, or molds for rapid thermal cycling, our expertise ensures reliability and efficiency for your laboratory.
Contact us today to discuss how our graphite solutions can enhance your high-temperature processes and reduce operational costs.
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Large Vertical Graphite Vacuum Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- How does a graphite furnace work? Achieve Extreme Temperatures in a Pure Environment
- What is responsible for electrical conductivity in graphite? Unlocking the Power of Delocalized Electrons
- What are the disadvantages of graphite furnace? Key Limitations and Operational Costs
- How does an induction graphitization furnace facilitate the transformation of unburned carbon into synthetic graphite?
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity
- What is the application of graphite furnace? Essential for High-Temp Material Processing & Synthesis
- What are the interferences of graphite furnace? Overcome Matrix & Spectral Issues for Accurate GFAAS
- What is the graphite furnace technique? Achieve Extreme Temperatures for Advanced Materials



















