At its core, argon's importance stems from a single, powerful property: its chemical inertness. This inability to react with other elements makes it an invisible workhorse across dozens of industries. From preserving priceless historical documents and enabling high-tech manufacturing to improving the energy efficiency of our homes and performing life-saving surgeries, argon's value lies in what it doesn't do.
The real question isn't just what argon is used for, but why it is so uniquely suited for these tasks. The answer is a combination of its extreme chemical stability, its specific physical properties like low thermal conductivity, and its predictable behavior under an electrical charge.
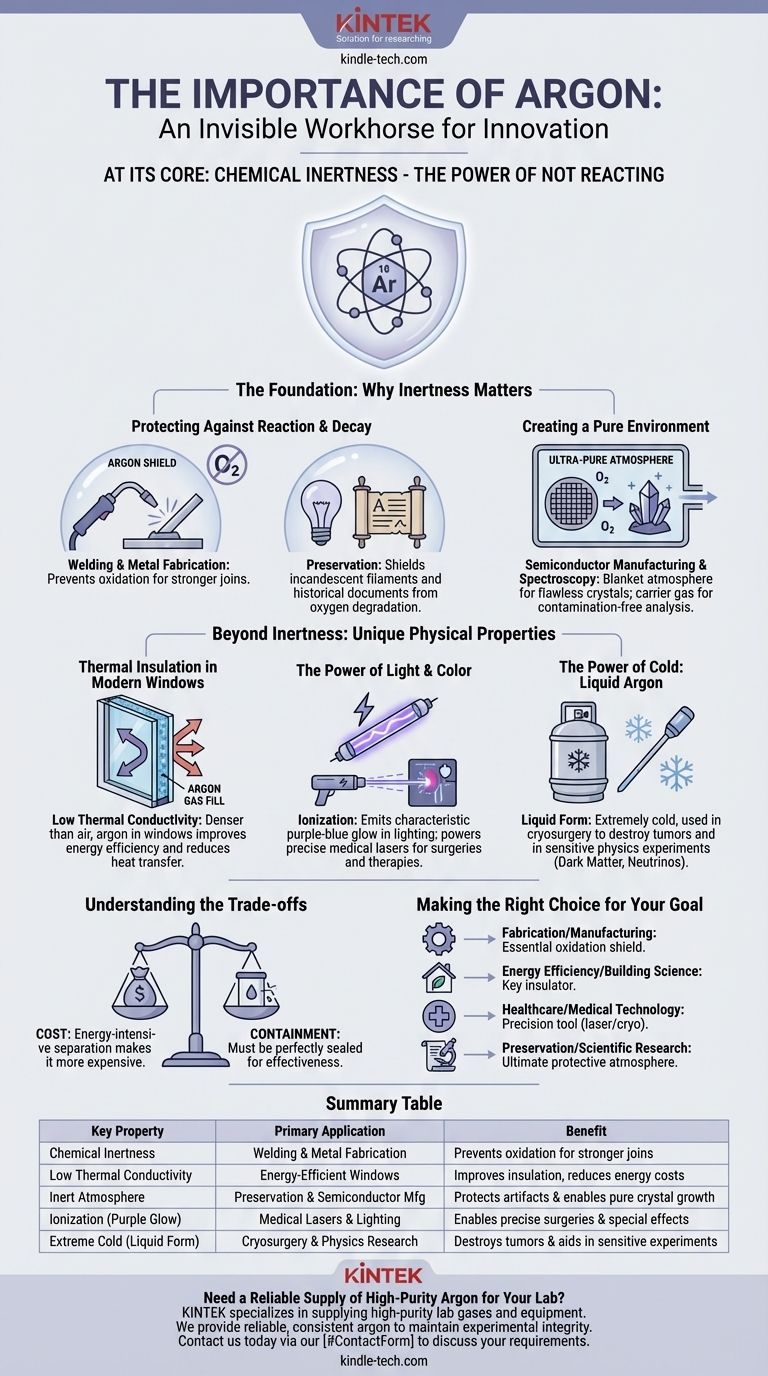
The Foundation: Why Inertness Matters
The vast majority of argon's applications derive from its status as a noble gas. It does not readily form chemical bonds, making it a perfect protective shield in sensitive environments.
Protecting Against Reaction and Decay
In many processes, the presence of reactive gases like oxygen is a problem. Argon provides a solution by displacing the air and creating a safe, non-reactive bubble.
This is critical in high-temperature applications like welding and metal fabrication, where it prevents the oxidation of hot metal, ensuring a stronger and cleaner join.
Similarly, in incandescent light bulbs, a pure argon atmosphere prevents the tungsten filament from burning out, dramatically extending the bulb's lifespan. This same principle allows it to preserve ancient documents by shielding them from oxygen-driven degradation.
Creating a Pure Environment
Beyond simply preventing reactions, argon is used to create the ultra-pure atmospheres required for advanced manufacturing and science.
It serves as a blanket atmosphere for growing flawless silicon and germanium crystals for the semiconductor industry. It is also used as a carrier gas in spectroscopy, ensuring that analytical results are not skewed by contamination from reactive gases.
Beyond Inertness: Unique Physical Properties
While its inertness is its primary feature, argon's physical characteristics open up another range of important applications, from thermal insulation to medical technology.
Thermal Insulation in Modern Windows
Argon is significantly denser than air and has low thermal conductivity. This makes it an excellent insulator.
In double- and triple-glazed windows, the space between the panes is filled with argon gas. This slows the transfer of heat, keeping homes warmer in the winter and cooler in the summer, which improves energy efficiency and reduces utility costs.
The Power of Light and Color
When an electric current is passed through it, argon emits a characteristic purple-blue glow. This property is used in "neon" lighting and for creating special effects in laser shows.
More critically, argon is the basis for argon ion lasers. These lasers are staples in forensic medicine, high-speed printing, and medical procedures like retinal phototherapy for diabetic patients.
The Power of Cold: Liquid Argon
In its liquid form, argon is extremely cold. This property is harnessed for cryosurgery, a medical procedure used to freeze and destroy tumors and other abnormal tissues with minimal damage to surrounding areas.
It's also used in highly sensitive physics experiments, including the search for dark matter and the study of neutrinos, where its purity and low temperature are essential.
Understanding the Trade-offs
No material is perfect for every application. Understanding argon's limitations is key to appreciating its role.
The Primary Factor: Cost
The most significant drawback of using argon is its cost. While abundant in the atmosphere, the process of separating it from air through fractional distillation is energy-intensive. This makes it more expensive than other potential gases, meaning it is typically reserved for applications where its specific properties are a necessity.
The Need for Containment
As a gas, argon must be perfectly sealed to be effective in applications like insulated windows or light bulbs. Any leak degrades its performance, rendering it no better than the air it replaced. This requirement adds a layer of manufacturing complexity and a potential point of failure.
Making the Right Choice for Your Goal
Argon is not a one-size-fits-all solution; its value is defined by the specific problem you are trying to solve.
- If your primary focus is fabrication or manufacturing: Argon is an essential shield, preventing oxidation in high-temperature processes like welding to ensure material integrity.
- If your primary focus is energy efficiency and building science: Argon is a key insulator in high-performance windows, offering a clear upgrade over air for reducing heat transfer.
- If your primary focus is healthcare and medical technology: Argon is a precision tool, used in lasers to repair tissue and in cryosurgery to destroy unhealthy cells with high accuracy.
- If your primary focus is preservation or scientific research: Argon's inert nature provides the ultimate protective atmosphere for priceless artifacts and the stable environment needed for cutting-edge experiments.
Ultimately, understanding argon's properties reveals how a simple, non-reactive element becomes an indispensable tool for innovation and protection.
Summary Table:
| Key Property | Primary Application | Benefit |
|---|---|---|
| Chemical Inertness | Welding & Metal Fabrication | Prevents oxidation for stronger joins |
| Low Thermal Conductivity | Energy-Efficient Windows | Improves insulation, reduces energy costs |
| Inert Atmosphere | Preservation & Semiconductor Manufacturing | Protects artifacts and enables pure crystal growth |
| Ionization (Purple Glow) | Medical Lasers & Lighting | Enables precise surgeries and special effects |
| Extreme Cold (Liquid Form) | Cryosurgery & Physics Research | Destroys tumors and aids in sensitive experiments |
Need a Reliable Supply of High-Purity Argon for Your Lab?
Argon's inert properties are essential for protecting sensitive processes and ensuring accurate results in the laboratory. Whether you're conducting research, performing analysis, or developing new materials, the quality of your inert gas is critical.
KINTEK specializes in supplying high-purity lab gases and equipment. We understand the precise demands of laboratory work and provide the reliable, consistent argon supply you need to maintain the integrity of your experiments and applications.
Contact us today via our [#ContactForm] to discuss your specific requirements and discover how KINTEK can support your laboratory's success with our trusted products and expertise.
Visual Guide
Related Products
- Custom Boron Nitride (BN) Ceramic Parts
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
People Also Ask
- How does the atmosphere affect sintering? Master Final Part Quality with Controlled Atmospheres
- What are the safety precautions for argon welding? Essential Guide to Protecting Against UV, Fumes, Shock, and Asphyxiation
- What is the atmosphere of annealing? Protect Your Metal from Oxidation & Decarburisation
- Why hydrogen is used in annealing furnace? Achieve Superior Purity and Thermal Control
- Why is atmosphere control essential during the pyrolysis of silicone composites? Ensure High-Density Ceramic Integrity
- What is the function of the inert atmosphere in sintering nickel-alumina? Achieve High-Purity Composite Bonding
- How does a high-temperature atmosphere furnace simulate service environments for evaluating CMAS corrosion resistance?
- How does a lab atmosphere furnace help synthesize PdCuAu alloys? Optimize Your Material Research Results



















