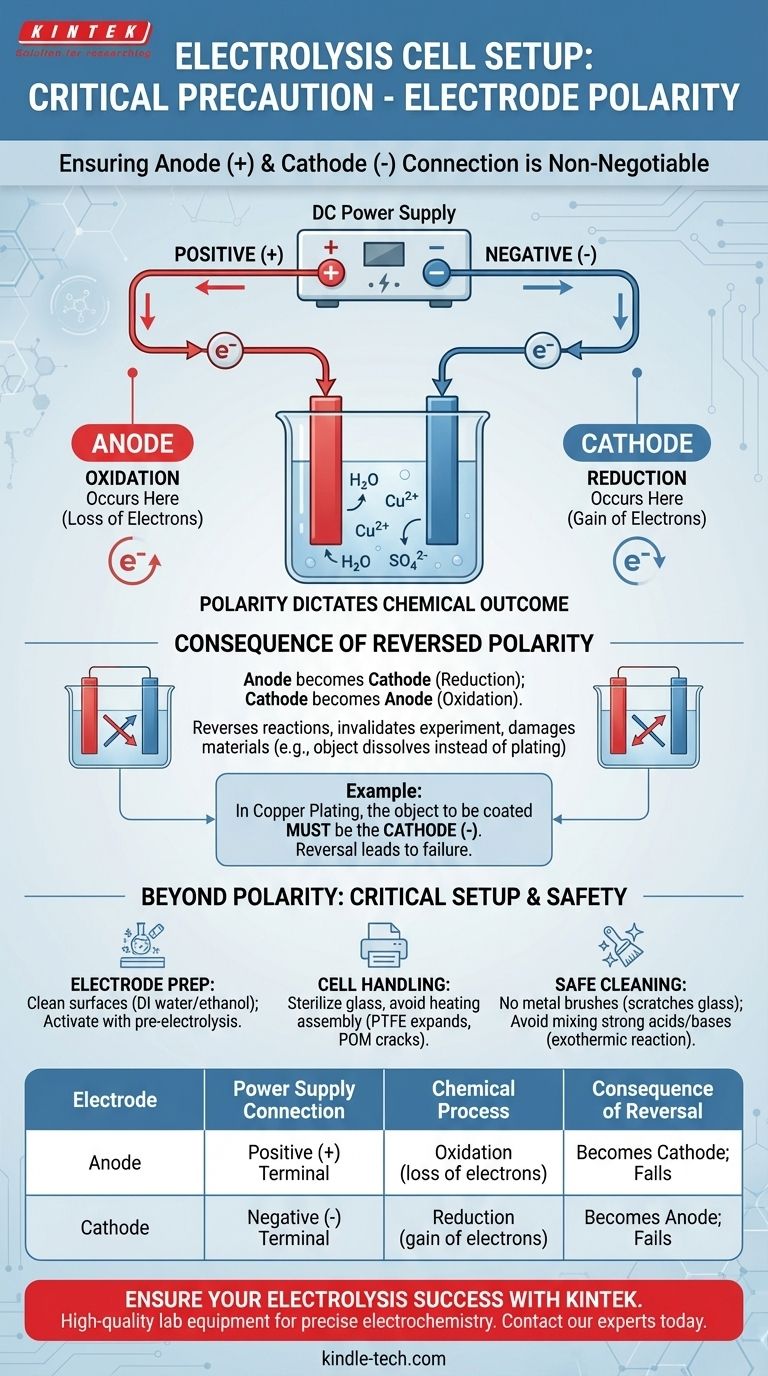
The most critical precaution regarding electrode polarity is to ensure the anode is connected to the positive (+) terminal of your DC power supply and the cathode is connected to the negative (-) terminal. This assignment is absolute and determines the entire chemical outcome of your experiment. Reversing this connection will reverse the intended reactions at each electrode, leading to incorrect products and potential damage to your materials.
Correctly setting the electrode polarity is not just a procedural step; it is the fundamental act of defining which chemical reaction—oxidation or reduction—will occur at each surface. Getting this wrong invalidates the entire purpose of the electrolysis.

Why Polarity is Non-Negotiable in Electrolysis
An electrolytic cell uses electrical energy to drive a non-spontaneous chemical reaction. The polarity of the power source dictates which electrode forces a chemical species to lose electrons (oxidation) and which forces a species to gain them (reduction).
The Role of the Anode (Oxidation)
The anode is, by definition, the electrode where oxidation occurs. To force a substance to lose its electrons, you must connect this electrode to the positive (+) terminal of the DC power supply. The positive terminal actively pulls electrons away from the anode and into the external circuit.
The Role of the Cathode (Reduction)
The cathode is the electrode where reduction occurs. To force a substance to gain electrons, you must connect this electrode to the negative (-) terminal of the power supply. The negative terminal actively pushes electrons from the external circuit onto the cathode's surface, where they can be accepted by the electrolyte.
The Consequence of Reversed Polarity
If you inadvertently swap the connections, the electrode you intended to be the anode becomes the cathode, and vice versa. The fundamental reactions are reversed at each location.
For example, in copper plating, you want to deposit copper (Cu²⁺ + 2e⁻ → Cu) onto an object. The object must be the cathode (negative terminal). If you connect it to the positive terminal, it will become the anode and may begin to dissolve instead.
Beyond Polarity: Critical Setup and Safety Precautions
A successful experiment depends on more than just correct polarity. The physical and chemical state of your cell and electrodes is equally important for obtaining reliable and safe results.
Electrode Preparation and Activation
Always clean your electrode surfaces before an experiment, typically with deionized water or ethanol, to remove any organic impurities or dust.
For some materials, a brief "pre-electrolysis" in the electrolyte can help activate the surface by removing any passive oxide layers that could interfere with your primary reaction.
Cell Handling and Material Limits
The glass components of a cell can often be sterilized under high-pressure steam (121℃), but the entire assembly should never be heated.
Materials like PTFE (Teflon) can expand permanently when heated, and POM (polyoxymethylene) can crack. These thermal limitations are critical to respect to avoid damaging the cell.
Safe Cleaning Procedures
Never use metal brushes to clean the interior of a glass cell, as they can create microscopic scratches that weaken the glass and create sites for contamination.
Avoid mixing strong acids and bases (like nitric acid and sodium hydroxide) for cleaning purposes. This can cause a violent, heat-generating (exothermic) reaction that is extremely dangerous.
Making the Right Choice for Your Goal
Always double-check your connections against your experimental goal. The role of each electrode is defined by what you need to accomplish.
- If your primary focus is electroplating or deposition: The object you want to coat must be the cathode, connected to the negative (-) terminal.
- If your primary focus is generating specific gases from an electrolyte: Remember that hydrogen (from H⁺ reduction) is produced at the cathode (-), and oxygen (from H₂O or OH⁻ oxidation) is produced at the anode (+).
- If your primary focus is electrosynthesis or purification: The polarity determines which starting material is oxidized at the anode (+) and which is reduced at the cathode (-), so it must be set according to your desired reaction pathway.
Ultimately, correct polarity ensures you are driving the specific chemical change you intend to study or produce.
Summary Table:
| Electrode | Power Supply Connection | Chemical Process | Consequence of Reversal |
|---|---|---|---|
| Anode | Positive (+) Terminal | Oxidation (loss of electrons) | Becomes the cathode; intended oxidation reaction fails |
| Cathode | Negative (-) Terminal | Reduction (gain of electrons) | Becomes the anode; intended reduction reaction fails |
Ensure your electrolysis experiments succeed every time. Correct polarity is just one aspect of proper lab setup. KINTEK specializes in high-quality lab equipment and consumables, providing the reliable tools you need for precise electrochemistry, synthesis, and analysis. Contact our experts today to discuss your specific laboratory requirements and discover how our solutions can enhance your research efficiency and safety.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity
- How should an all-quartz electrolytic cell and its components be maintained for long-term use? A Guide to Maximizing Equipment Lifespan
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis



















