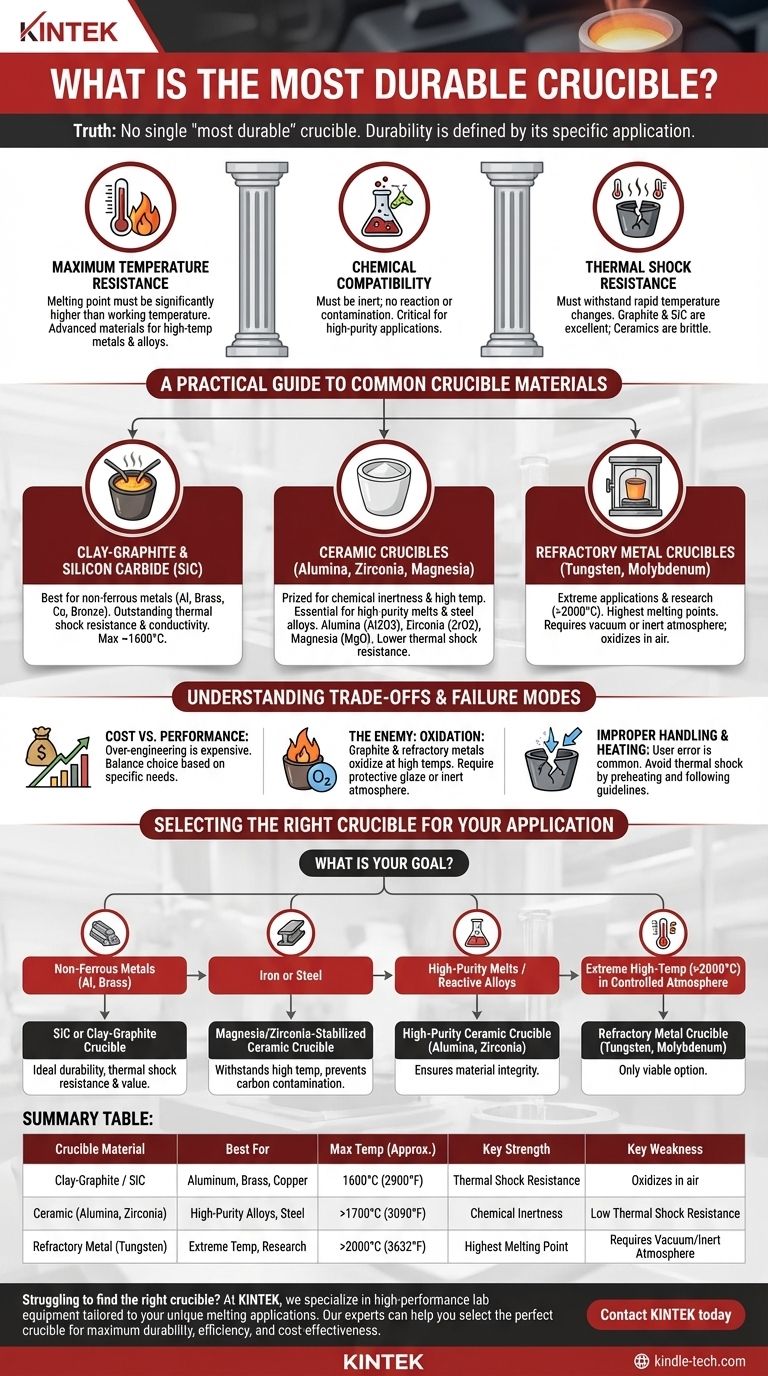
In truth, there is no single "most durable" crucible. The durability of a crucible is not an intrinsic quality but is defined entirely by its specific application. A crucible that excels at melting aluminum would be instantly destroyed by molten steel, while one designed for steel might be chemically unsuitable for a high-purity aerospace alloy. The most durable crucible is the one whose material properties are correctly matched to the task at hand.
The concept of "durability" must be broken down into three critical factors: maximum temperature resistance, chemical compatibility with the material being melted, and resistance to thermal shock. True durability is achieved only when a crucible satisfies all three requirements for your specific process.

The Three Pillars of Crucible Durability
To select a crucible that will last, you must move beyond the simple question of "what's toughest?" and instead analyze the distinct challenges your process will present.
### Maximum Temperature Resistance
This is the most straightforward factor. The crucible material must have a melting point significantly higher than the working temperature of the material you are melting.
Materials are often grouped by their temperature capabilities. Clay-graphite and silicon carbide are excellent for most non-ferrous metals, while advanced ceramics and refractory metals are required for high-temperature steels, platinum group metals, and specialty alloys.
### Chemical Compatibility
A crucible can fail chemically long before it fails thermally. The crucible must be chemically inert, meaning it must not react with, dissolve into, or otherwise contaminate the molten material it holds.
For example, using a graphite crucible to melt steel is a critical error. The molten iron will readily absorb carbon from the crucible, fundamentally changing the steel's properties and degrading the crucible itself. For high-purity applications, an inert ceramic like alumina or zirconia is often required.
### Thermal Shock Resistance
Thermal shock is the stress a material endures when its temperature changes rapidly, causing it to crack. A crucible must be able to withstand being heated quickly and, in some cases, tolerate the introduction of cooler solid material for melting.
Materials like graphite and silicon carbide have excellent thermal shock resistance due to their high thermal conductivity, which prevents sharp temperature gradients from forming. In contrast, many ceramic materials are more brittle and require careful, slow pre-heating and cooling cycles to prevent catastrophic failure.
A Practical Guide to Common Crucible Materials
Understanding the fundamental properties of each material type is the key to making an informed decision.
### Clay-Graphite and Silicon Carbide (SiC)
These are the workhorses for hobbyists and foundries working with non-ferrous metals like aluminum, brass, copper, and bronze. The graphite provides outstanding thermal conductivity and shock resistance, while the clay or silicon carbide binders add strength and oxidation resistance.
They offer the best all-around balance of performance, thermal shock resistance, and cost-effectiveness for applications below 1600°C (2900°F).
### Ceramic Crucibles (Alumina, Zirconia, Magnesia)
Ceramic crucibles are prized for their chemical inertness and high-temperature capabilities, making them essential for high-purity melts or when working with reactive metals.
Alumina (Al2O3) is a common choice for its excellent performance and reasonable cost. Zirconia (ZrO2) and Magnesia (MgO) are used for even higher temperatures, such as melting platinum or steel alloys, where contamination must be minimized. Their primary weakness is a lower resistance to thermal shock compared to graphite-based crucibles.
### Refractory Metal Crucibles (Tungsten, Molybdenum)
These are highly specialized crucibles for the most extreme applications, such as research and semiconductor manufacturing. Tungsten has the highest melting point of any metal (3422°C / 6192°F) and is used for ultra-high temperature processes.
However, these metals oxidize catastrophically in open air at high temperatures. They can only be used in a vacuum or a fully inert gas atmosphere furnace, which adds significant complexity and cost to the process.
Understanding the Trade-offs and Failure Modes
The "best" choice is always a balance. Being aware of the limitations is just as important as knowing the strengths.
### Cost vs. Performance
A specialized zirconia crucible may be technically superior for melting brass, but a silicon carbide crucible will perform the task perfectly well for a fraction of the cost. Over-engineering your choice is a common and expensive mistake.
### The Enemy: Oxidation
Graphite and refractory metal crucibles are highly susceptible to oxidation. At high temperatures, oxygen in the air will burn them away, drastically reducing their lifespan. Graphite crucibles are often made with a protective glaze to mitigate this, but careful handling is still required.
### Improper Handling and Heating
The most common cause of crucible failure is user error. Dropping cold metal into a red-hot crucible can cause a thermal shock fracture. Likewise, heating a ceramic crucible too quickly will crack it before it ever sees molten metal. Always follow the manufacturer's guidelines for preheating.
Selecting the Right Crucible for Your Application
Use your specific goal to guide your final choice.
- If your primary focus is melting common non-ferrous metals like aluminum or brass: A silicon carbide or clay-graphite crucible offers the ideal combination of durability, thermal shock resistance, and value.
- If your primary focus is melting iron or steel: A specialized magnesia- or zirconia-stabilized ceramic crucible is necessary to withstand the high temperatures and prevent carbon contamination.
- If your primary focus is high-purity melts or reactive alloys: An appropriate high-purity ceramic crucible, such as alumina or zirconia, is the correct choice to ensure the integrity of your material.
- If your primary focus is extreme high-temperature work (>2000°C) in a controlled atmosphere: A refractory metal crucible, such as tungsten or molybdenum, is the only viable option.
Ultimately, the most durable crucible is the one engineered to meet the specific thermal, chemical, and physical demands of your unique process.
Summary Table:
| Crucible Material | Best For | Max Temp (Approx.) | Key Strength | Key Weakness |
|---|---|---|---|---|
| Clay-Graphite / SiC | Aluminum, Brass, Copper | 1600°C (2900°F) | Thermal Shock Resistance | Oxidizes in air |
| Ceramic (Alumina, Zirconia) | High-Purity Alloys, Steel | >1700°C (3090°F) | Chemical Inertness | Low Thermal Shock Resistance |
| Refractory Metal (Tungsten) | Extreme Temp, Research | >2000°C (3632°F) | Highest Melting Point | Requires Vacuum/Inert Atmosphere |
Struggling to find the right crucible for your lab's specific needs? At KINTEK, we specialize in providing high-performance lab equipment and consumables tailored to your unique melting applications. Our experts can help you select the perfect crucible material—whether you're working with non-ferrous metals, high-purity alloys, or extreme-temperature processes—ensuring maximum durability, efficiency, and cost-effectiveness. Don't let the wrong crucible compromise your results. Contact KINTEK today for a personalized consultation and discover how our solutions can enhance your laboratory's performance.
Visual Guide
Related Products
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- Why are high-purity ceramic crucibles necessary for Pt/Pd alloys? Ensure Purity in High-Temperature Synthesis
- What role does a graphite crucible with a tight-fitting lid play in smelting? Master the Reductive Micro-Environment
- How do high-temperature reactors and ceramic crucibles facilitate LaFeO3 perovskite coatings? High-Purity Synthesis Guide
- What are the different sizes of crucibles? A Guide from Jewelry to Industrial Scales
- Why are alumina crucibles recommended over quartz crucibles for liquid aluminum? Ensure Experimental Accuracy
- What is the use of crucible furnace? Unlock Versatile Melting for Metals & Materials
- What are the properties of a good crucible? Essential Guide to High-Temperature Performance
- Why use MgO crucibles for sintering LLZTO ceramic pellets? Ensure Purity and High Ionic Conductivity



















