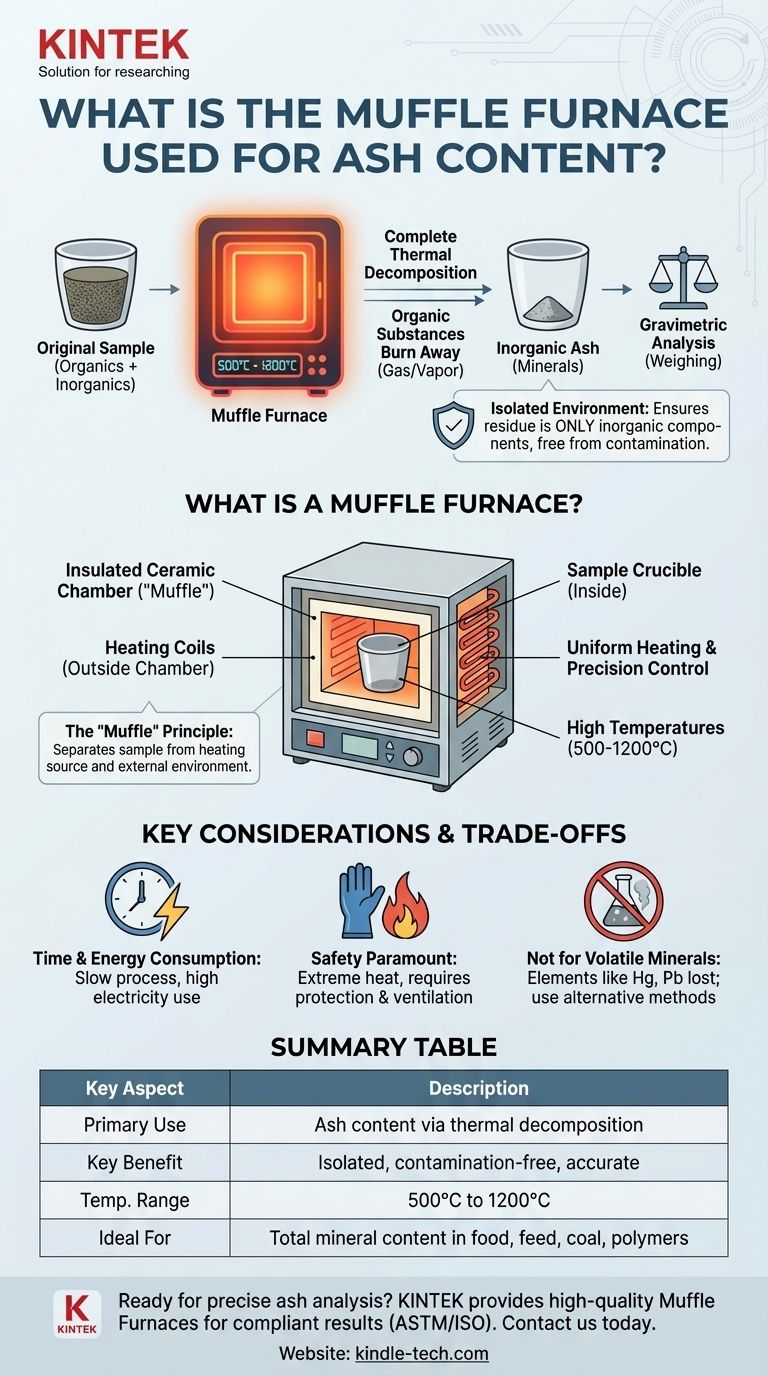
In analytical testing, a muffle furnace is used for ash content determination by heating a sample to a very high, specific temperature until all organic substances burn away. This process of complete thermal decomposition, known as ashing or incineration, leaves behind only the inorganic, non-combustible materials (the "ash"), which can then be weighed for analysis.
The muffle furnace is more than just a powerful oven. Its critical function is to provide an extremely hot, precisely controlled, and completely isolated environment, ensuring that the residue left after heating consists only of the sample's own inorganic components, free from any external contamination.

What is a Muffle Furnace?
A muffle furnace is a standard piece of laboratory equipment designed for high-temperature applications. It's also known as a chamber or box furnace due to its simple, insulated box design.
A High-Temperature, Isolated Chamber
The core of the device is a chamber, or "muffle," that is insulated with dense ceramic material. Electric coils outside this chamber heat it through radiation, allowing it to reach temperatures far beyond a conventional oven, often between 500°C and 1200°C (932°F and 2192°F).
The Principle of the "Muffle"
The term "muffle" refers to the separation of the sample from the heating source and the external environment. In modern electric furnaces, this means the heating coils are not exposed to the sample, preventing contamination and protecting the coils from any corrosive fumes the sample might release.
This isolation ensures that the only thing affecting the sample is pure, controlled heat.
Precision and Control
Unlike a simple burner, a muffle furnace provides uniform heating and precise digital temperature control. This is critical for scientific and industrial procedures where results must be repeatable and adhere to strict standards (such as ASTM or ISO methods).
The Role of the Furnace in Ash Content Analysis
Determining ash content is a form of gravimetric analysis—a method that relies on measuring mass. The muffle furnace is the essential tool that makes this measurement accurate.
The Goal: Complete Combustion
The purpose of ashing is to completely remove all organic matter from a sample. Organic compounds are primarily made of carbon, hydrogen, oxygen, and nitrogen, all of which turn into gas (like carbon dioxide and water vapor) and dissipate when burned at high temperatures.
Why High Temperature is Essential
To ensure every last bit of organic material is burned away, temperatures of 550°C or higher are typically required. This process, often called "dry ashing," guarantees that what remains is not partially charred material, but true inorganic residue.
Leaving Only the Inorganic Ash
After the combustion process is complete, the only thing left in the crucible is the ash. This substance represents the total mineral content of the original sample—things like calcium, potassium, magnesium, and other non-combustible elements.
By weighing the sample before and after ashing, you can calculate the percentage of inorganic material it contains with high accuracy. This is a common quality metric for food, animal feed, coal, and plastics.
Understanding the Key Considerations
While highly effective, using a muffle furnace for ashing involves practical considerations and trade-offs.
Time and Energy Consumption
Ashing is a slow process that can take several hours to ensure complete combustion. Because they generate extreme heat, muffle furnaces also consume a significant amount of electricity.
Safety is Paramount
These devices operate at incredibly high temperatures. The insulated doors and walls become extremely hot, and operators must always use heat-resistant gloves and tongs to handle crucibles. Proper ventilation is also necessary to remove fumes produced during combustion.
Not Suitable for Volatile Minerals
Dry ashing in a muffle furnace is not appropriate for samples containing volatile inorganic elements, such as mercury, lead, or selenium. The high temperatures would cause these elements to vaporize along with the organic matter, leading to an inaccurate, understated ash content.
Making the Right Choice for Your Analysis
Properly applying this tool requires understanding your analytical goal.
- If your primary focus is determining the total mineral content of a stable sample (like flour, polymers, or coal): The muffle furnace is the standard and most reliable instrument for complete thermal decomposition.
- If your primary focus is quality control following an industry standard (like ASTM D3174 for coal ash): The muffle furnace provides the precise, repeatable high-temperature environment required for compliance.
- If your primary focus is analyzing for volatile metals that would be lost to heat: You must use an alternative method, such as wet ashing with acids, to avoid inaccurate results.
Understanding the function of the muffle furnace is fundamental to achieving accurate and defensible results in gravimetric analysis.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Use | Ash content determination via complete thermal decomposition (ashing). |
| Key Benefit | Isolated, contamination-free heating for accurate, repeatable results. |
| Typical Temperature Range | 500°C to 1200°C (932°F to 2192°F). |
| Ideal For | Analyzing total mineral content in food, feed, coal, polymers, and more. |
Ready to achieve precise and compliant ash content analysis in your lab?
KINTEK specializes in high-quality lab equipment, including reliable muffle furnaces designed for accurate gravimetric analysis. Our furnaces provide the precise temperature control and isolated environment you need for dependable results, helping you meet industry standards like ASTM and ISO.
Contact us today using the form below to find the perfect solution for your laboratory's needs and enhance your analytical testing capabilities.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the inside material of the muffle furnace? Discover the Refractory Core for High-Temp Precision
- What are the acceptance criteria for muffle furnace? Ensure Safety, Performance & Success
- How hot can a muffle furnace get? Find the Right Temperature for Your Lab
- What are the safety precautions for muffle furnace? A Complete Guide to Safe High-Temperature Operation
- How do you use the muffle furnace? Master Safe and Precise High-Temperature Processing



















