The pressed pellet technique is a standard method for preparing solid samples for analysis by infrared (IR) spectroscopy. It involves intimately mixing a finely ground solid sample with a larger quantity of an IR-transparent salt, most commonly potassium bromide (KBr), and then compressing this mixture under high pressure to form a thin, transparent disc suitable for analysis.
The core objective of this technique is to suspend the solid analyte in a non-absorbing, non-scattering medium. By creating a high-quality, transparent KBr pellet, you can obtain a clean IR spectrum of the solid itself, free from the interference and light scattering that would occur if you tried to analyze the raw powder directly.

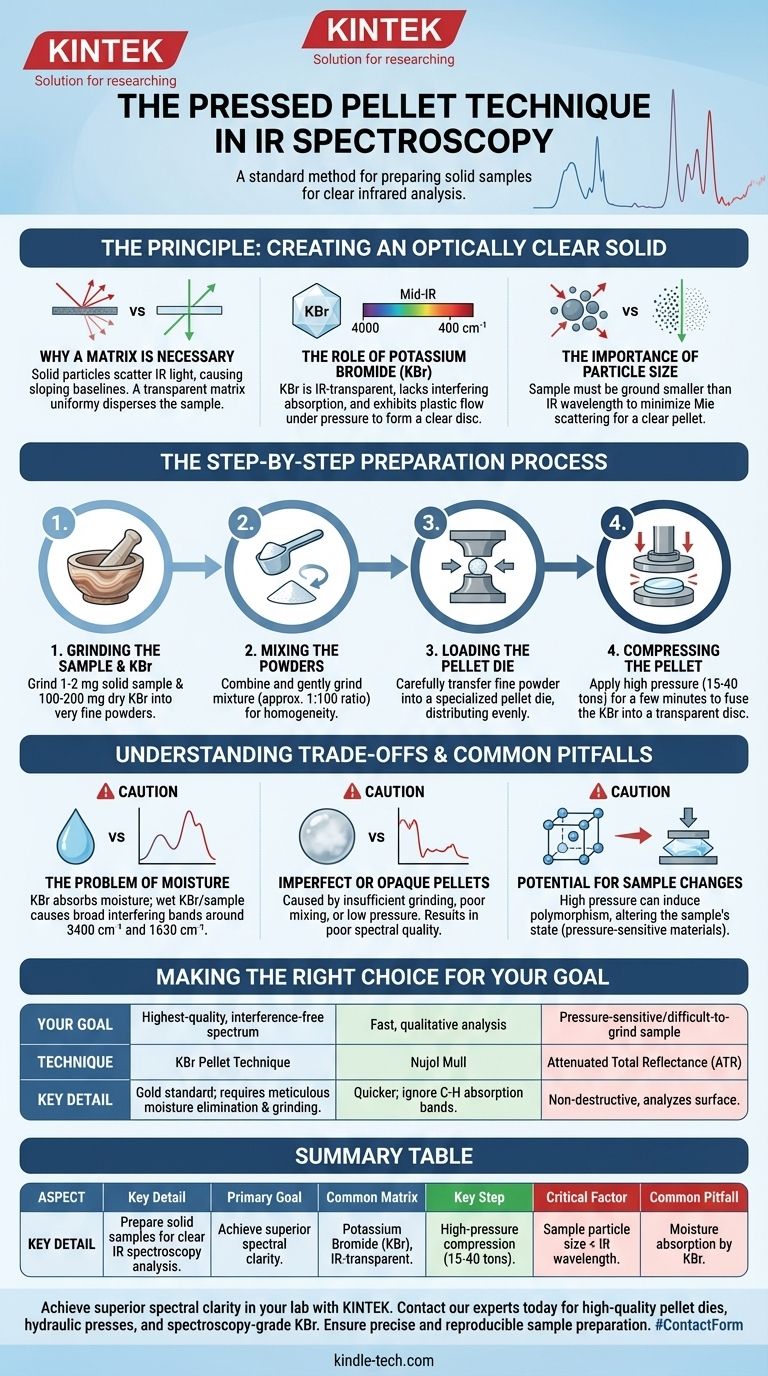
The Principle: Creating an Optically Clear Solid
Why a Matrix is Necessary
Solid particles naturally scatter infrared light, a phenomenon that results in a sloping baseline and poor-quality spectra. To get a useful spectrum, the sample must be uniformly dispersed in a matrix that is transparent to IR radiation and has a similar refractive index.
The Role of Potassium Bromide (KBr)
Potassium bromide is the most widely used material for this purpose. It is chosen because it is transparent across the entire mid-IR region (typically 4000 cm⁻¹ to 400 cm⁻¹), meaning it does not have its own absorption bands that would interfere with the sample's spectrum. Under pressure, KBr also exhibits plastic flow, allowing it to form a glassy, transparent disc that encapsulates the sample particles.
The Importance of Particle Size
For the technique to be successful, the sample particles must be ground to a size smaller than the wavelength of the IR radiation being used. This minimizes light scattering (known as Mie scattering) and is critical for producing a clear pellet and a flat spectral baseline.
The Step-by-Step Preparation Process
Step 1: Grinding the Sample and KBr
First, a very small amount of the solid sample (typically 1-2 mg) is ground into an exceptionally fine powder, usually with an agate mortar and pestle. Spectroscopy-grade KBr (around 100-200 mg) must also be completely dry, often by heating it in an oven, as it readily absorbs water from the atmosphere.
Step 2: Mixing the Powders
The ground sample is combined with the dry KBr. A common ratio is approximately 1 part sample to 100 parts KBr. This mixture must be ground together gently but thoroughly to ensure the sample is homogeneously distributed throughout the salt.
Step 3: Loading the Pellet Die
The fine powder mixture is carefully transferred into a specialized pellet die. This consists of a hollow barrel and two polished steel bolts or anvils that fit inside it. The powder is distributed evenly on the surface of one anvil before the second is placed on top.
Step 4: Compressing the Pellet
The loaded die is placed into a hydraulic press. A very high pressure, typically between 15 and 40 tons, is applied for a few minutes. This immense pressure causes the KBr to fuse into a solid, transparent or translucent disc with the sample particles trapped inside. The pellet is then carefully removed from the die and placed in the spectrometer's sample holder.
Understanding the Trade-offs and Common Pitfalls
The Problem of Moisture
KBr is highly hygroscopic, meaning it easily absorbs water from the air. If the KBr or the sample is not perfectly dry, water will be incorporated into the pellet. This is the most common problem and results in broad, interfering absorption bands in the spectrum around 3400 cm⁻¹ and 1630 cm⁻¹, which can obscure important features of your sample.
Imperfect or Opaque Pellets
A cloudy or opaque pellet is a sign of failure. This can be caused by several factors:
- Insufficient grinding: Large sample particles will scatter light.
- Poor mixing: Inhomogeneous distribution of the sample leads to cloudiness.
- Insufficient pressure: The KBr will not fuse properly if the pressure is too low.
An opaque pellet will produce a very poor spectrum with a severely sloping baseline and weak peaks.
Potential for Sample Changes
The high pressure used to form the pellet can sometimes induce a change in the sample's crystalline form (polymorphism). This means the resulting spectrum may not represent the sample in its original state. This is a rare but important consideration for pressure-sensitive materials.
Making the Right Choice for Your Goal
- If your primary focus is obtaining the highest-quality, interference-free spectrum: The KBr pellet technique is the gold standard, provided you take meticulous care to eliminate moisture and ensure proper grinding.
- If your primary focus is a fast, qualitative analysis: A Nujol mull (grinding the sample in mineral oil) is a quicker alternative, but you must be prepared to identify and ignore the oil's own C-H absorption bands in the spectrum.
- If your sample is pressure-sensitive or difficult to grind: Consider using an Attenuated Total Reflectance (ATR) accessory, a modern, non-destructive technique that analyzes the surface of a solid with minimal preparation.
Mastering the pressed pellet technique is a valuable laboratory skill that provides unparalleled clarity for the structural analysis of solid materials via infrared spectroscopy.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Primary Goal | Prepare solid samples for clear IR spectroscopy analysis. |
| Common Matrix | Potassium Bromide (KBr), IR-transparent. |
| Key Step | High-pressure compression (15-40 tons) to form a transparent disc. |
| Critical Factor | Sample particle size must be smaller than IR wavelength. |
| Common Pitfall | Moisture absorption by KBr, leading to spectral interference. |
Achieve superior spectral clarity in your lab. The pressed pellet technique is fundamental for accurate material analysis. KINTEK specializes in providing the high-quality lab equipment and consumables—including reliable pellet dies, hydraulic presses, and spectroscopy-grade KBr—that your laboratory needs to master this method. Ensure your sample preparation is precise and reproducible. Contact our experts today to discuss your specific application and find the perfect solution for your IR spectroscopy workflow.
Visual Guide
Related Products
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- kbr pellet press 2t
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
People Also Ask
- What is a vacuum press machine? Harness Atmospheric Pressure for Perfect Lamination
- What is the function of a laboratory hydraulic press in the manufacturing of composite boards? Essential Densification
- What is a small hydraulic press used for? Unlock Precise, Powerful Force for Labs & Workshops
- What is the purpose of using a laboratory hydraulic press for LGVO synthesis? Achieve High-Purity Solid Electrolytes
- What products are made by press forging? High-Strength Components for Aerospace, Automotive & Energy
- Why is a laboratory hydraulic press essential for Cu-Mo alloy production? Maximize Green Body Strength and Density
- What is the construction of a hydraulic press? The Core Components Explained
- Why is a laboratory hydraulic press required during the preparation of Ti3AlC2 precursor pellets?



















