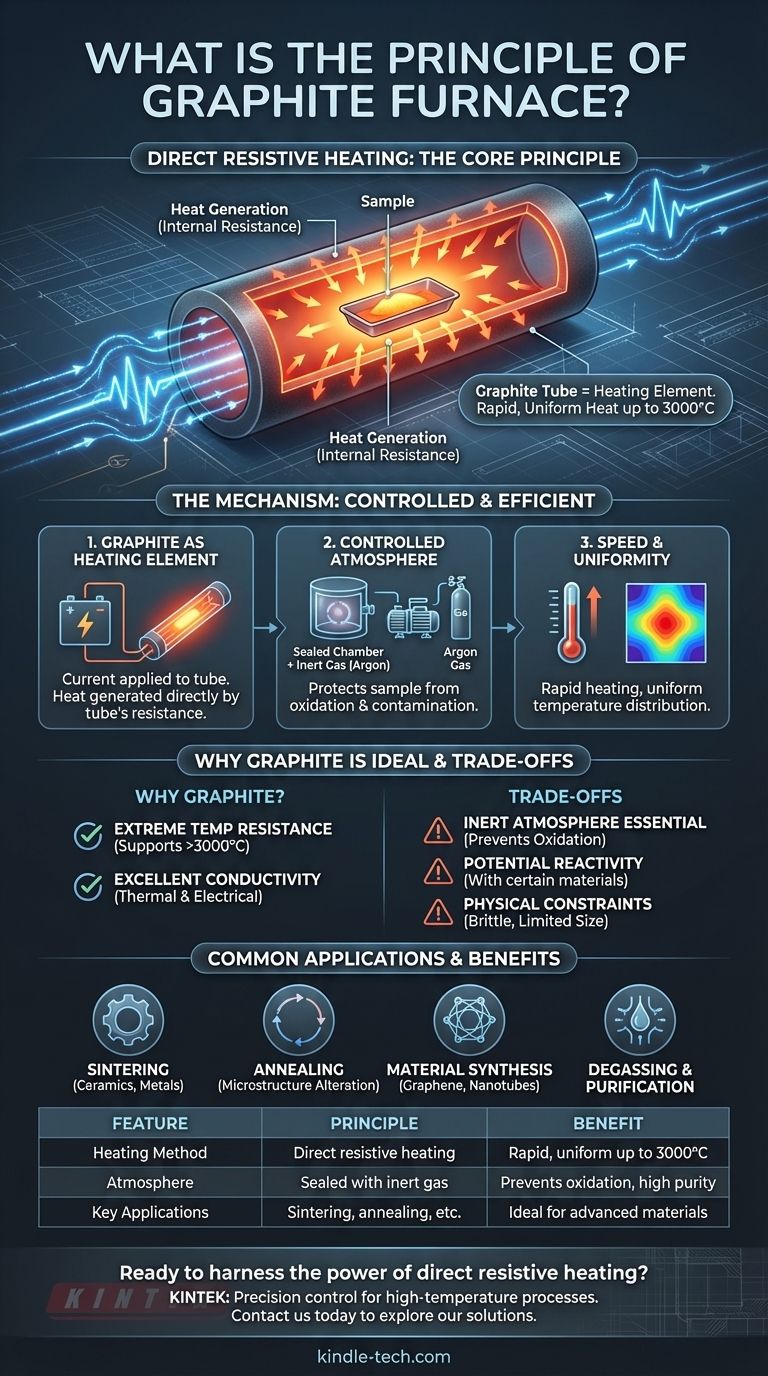
At its core, the principle of a graphite furnace is direct resistive heating. It works by passing a high electrical current through a graphite tube that contains the sample. The graphite's natural electrical resistance causes it to heat up rapidly and intensely, transferring that heat directly and uniformly to the material inside, all within a highly controlled atmosphere.
The central concept is using the furnace's main structural component—the graphite tube—as its own heating element. This simple, elegant design enables extremely high temperatures (up to 3000°C) with exceptional speed and uniformity, making it ideal for advanced material processing.

The Core Mechanism: Direct and Controlled Heating
The effectiveness of a graphite furnace comes from a few key design principles working in concert. It's not just about getting hot; it's about how it gets hot and the environment it creates.
The Graphite Tube as the Heating Element
The furnace's "hot zone" is constructed entirely of graphite. Instead of separate heating coils, the graphite tube that holds the sample is connected to an electrical power source.
When current is applied, the tube itself heats up due to its own internal resistance. This method is incredibly efficient, as the heat is generated exactly where it's needed, surrounding the sample.
Creating a Controlled Atmosphere
Graphite oxidizes (burns) at high temperatures in the presence of air. To prevent this and to protect the sample from contamination, the entire process takes place inside a sealed chamber.
This chamber is first evacuated to a vacuum and then typically backfilled with an inert gas, such as argon. This non-reactive atmosphere is critical for high-purity applications like degassing, sintering, and crystal growth.
Key Characteristics: Speed and Uniformity
Because the heat source is the tube itself, heating is extremely fast and evenly distributed around the sample. The graphite shields surrounding the tube help reflect thermal energy back, further enhancing temperature uniformity throughout the work area.
Why Graphite is the Ideal Material
The choice of graphite is not arbitrary. Its unique combination of properties makes it perfectly suited for this role.
Extreme Temperature Resistance
Graphite has one of the highest melting points of any material and maintains its structural integrity well past 3000°C. This allows the furnace to operate in a temperature range that most metals cannot withstand.
Excellent Thermal and Electrical Conductivity
Graphite conducts electricity well, which allows it to function as a resistive heater. Simultaneously, its high thermal conductivity ensures that the heat generated is spread evenly across its surface, preventing hot spots and ensuring the sample is heated uniformly.
Understanding the Trade-offs
While powerful, this technology is not without its specific operational requirements and limitations.
The Necessity of an Inert Atmosphere
The single most important operational requirement is the need for a vacuum or inert gas environment. Operating a graphite furnace in an oxygen-rich atmosphere will rapidly destroy the graphite elements.
Potential for Material Reactivity
At very high temperatures, carbon can react with certain samples. This must be considered when processing specific metals or ceramics to avoid unwanted carbide formation or material contamination.
Physical and Size Constraints
The usable work area in graphite tube furnaces is often limited, with typical diameters around four inches. The graphite components are also brittle and require careful handling during loading and maintenance.
Common Applications in Practice
The unique capabilities of the graphite furnace make it essential for a range of high-temperature processes.
Material Synthesis and Purification
The furnace's high-purity, controlled environment is ideal for processes like growing graphene or carbon nanotubes, degassing metals to remove impurities, and synthesizing advanced carbides.
Thermal Treatment and Processing
It is widely used for sintering ceramics and powdered metals into solid masses, annealing materials to alter their microstructure, brazing components, and performing graphitization to convert carbon precursors into crystalline graphite.
Making the Right Choice for Your Goal
Understanding the furnace's core principle helps you align its capabilities with your specific objective.
- If your primary focus is material purity and synthesis: The combination of a high-purity graphite hot zone and a controlled inert gas atmosphere is the most critical feature.
- If your primary focus is high-temperature thermal treatment: The furnace's ability to achieve rapid, uniform heating to 3000°C is its key advantage for processes like sintering or graphitizing.
- If your primary focus is process versatility: The furnace's ability to support numerous applications, from brazing to annealing to ceramic firing, makes it a powerful and flexible tool.
Ultimately, the graphite furnace provides an unparalleled method for achieving extreme temperatures with precision control in a pristine environment.
Summary Table:
| Feature | Principle | Benefit |
|---|---|---|
| Heating Method | Direct resistive heating of graphite tube | Rapid, uniform heating up to 3000°C |
| Atmosphere | Sealed chamber with inert gas (e.g., argon) | Prevents oxidation, ensures high-purity processing |
| Key Applications | Sintering, annealing, degassing, graphitization | Ideal for ceramics, metals, and advanced material synthesis |
| Material Suitability | High-purity graphite construction | Excellent thermal/electrical conductivity, extreme temperature resistance |
Ready to harness the power of direct resistive heating for your lab? KINTEK specializes in high-performance graphite furnaces and lab equipment, delivering the precise temperature control and purity you need for sintering, annealing, and material synthesis. Contact us today to explore how our solutions can elevate your high-temperature processes!
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- What is the temperature of a graphite furnace? Achieve Extreme Heat Up to 3000°C
- What is the temperature range of a graphite furnace? Unlock up to 3000°C for advanced materials processing.
- Can graphite withstand heat? Unlocking its extreme 3,600°C potential in inert environments
- What are the advantages of graphite? Unlock Superior Performance in High-Temperature Processes
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity



















