In essence, Chemical Vapor Deposition (CVD) is a process that transforms a gas into a solid coating. It works by introducing reactive precursor gases into a chamber containing a heated object, or substrate. The heat causes a chemical reaction, breaking down the gases and depositing a thin, solid film of the desired material onto the substrate's surface, while any waste products are removed.
The core principle of CVD is not simply "spraying" a coating, but rather growing a new solid layer on a surface through a precisely controlled chemical reaction. The quality, properties, and thickness of this new layer are determined by managing a delicate balance of temperature, pressure, and gas chemistry inside a reaction chamber.

The Fundamental Principles of CVD
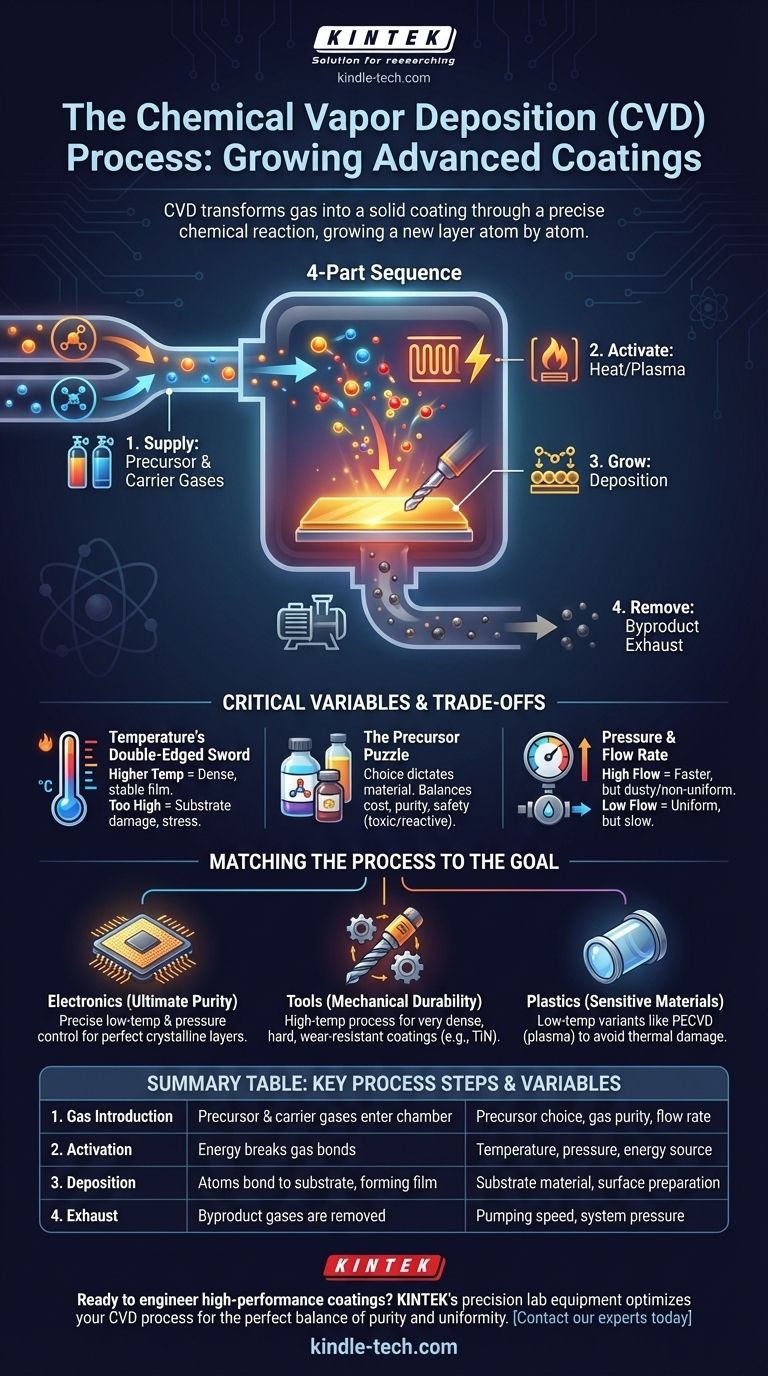
To truly understand CVD, it's best to think of it as a four-part sequence: supplying the ingredients, activating the reaction, growing the film, and removing the waste. Each stage is critical for producing a high-quality, uniform coating.
The Chamber and Substrate
The entire process takes place inside a sealed reaction chamber, which is often under a vacuum. This controlled environment is crucial to prevent contamination from air and other particles.
Inside the chamber is the substrate—the object to be coated. This can be anything from a silicon wafer for a microchip to a metal cutting tool or an optical lens.
The Precursor and Carrier Gases
The raw ingredients for the film are called precursor gases. These are volatile chemical compounds that contain the atoms needed for the final coating (e.g., silane gas, which contains silicon).
These precursors are often mixed with a carrier gas (like hydrogen or nitrogen). The carrier gas doesn't become part of the final coating; its job is to dilute the precursors and transport them uniformly over the substrate surface.
The Energy of Activation
The precursors will not react on their own. They require a significant amount of energy to break their chemical bonds.
Most commonly, this energy is supplied by heating the substrate to a very high temperature (thermal CVD). When the precursor gases hit the hot surface, they decompose. In some variations, this energy can be supplied by plasma (PECVD) or lasers (LCVD) to allow for lower processing temperatures.
Deposition and Film Growth
Once the precursor gases break down at the substrate surface, the desired atoms bond to the surface in a process called adsorption. They then arrange themselves into a stable, solid structure, forming a thin film.
This film grows layer by layer. The final thickness of the coating is precisely controlled by managing the duration of the process, the temperature, and the concentration of the precursor gases.
The Byproduct Exhaust
The chemical reactions that form the solid film also create unwanted byproducts, which are typically also in a gaseous state.
These waste gases, along with any unreacted precursor and carrier gas, are continuously pumped out of the chamber through an exhaust system. This step is vital for maintaining the chemical purity of the reaction and ensuring a high-quality final product.
Understanding the Critical Variables & Trade-offs
The success of a CVD process hinges on a delicate balance of several factors. Mismanaging any one of them can lead to poor film quality, non-uniform coatings, or damage to the substrate.
Temperature's Double-Edged Sword
Higher temperatures generally provide more energy for the reaction, leading to a denser, more pure, and more stable film. However, excessively high temperatures can damage heat-sensitive substrates, increase energy costs, and introduce thermal stress.
The Precursor Puzzle
The choice of precursor is fundamental; it dictates the material you can deposit. However, precursors vary widely in cost, purity, and safety. Some highly effective precursors are also highly toxic or pyrophoric (ignite spontaneously in air), requiring complex and expensive handling systems.
Pressure and Flow Rate
The pressure inside the chamber and the flow rate of the gases control the concentration of reactants at the substrate surface. High flow rates can increase the deposition speed but may lead to gas-phase reactions (forming dust) and non-uniform coatings. Low flow rates provide better uniformity but are much slower and less efficient.
Matching the Process to the Goal
Your specific objective dictates how these variables should be tuned. The "best" CVD process is the one that achieves the desired outcome for a specific application.
- If your primary focus is ultimate purity for electronics: You must use ultra-high-purity precursors and precisely control temperature and pressure to grow perfect crystalline layers on silicon wafers.
- If your primary focus is mechanical durability for tools: You will likely use a high-temperature process to create a very dense, hard, wear-resistant coating like titanium nitride (TiN) or a diamond-like carbon.
- If your primary focus is coating a temperature-sensitive material like plastic: You must use a low-temperature variant like Plasma-Enhanced CVD (PECVD), where plasma energy, not just heat, is used to activate the precursors.
By mastering the interplay of gas, heat, and pressure, CVD allows us to engineer materials with specific properties directly onto a surface, atom by atom.
Summary Table:
| CVD Process Step | Key Function | Critical Variables |
|---|---|---|
| 1. Gas Introduction | Precursor & carrier gases enter the chamber | Precursor choice, gas purity, flow rate |
| 2. Activation | Energy (heat/plasma) breaks gas bonds | Temperature, pressure, energy source |
| 3. Deposition | Atoms bond to substrate, forming a solid film | Substrate material, surface preparation |
| 4. Exhaust | Byproduct gases are removed from the chamber | Pumping speed, system pressure |
Ready to engineer high-performance coatings for your specific application?
Whether you're developing microelectronics, enhancing tool durability, or coating sensitive materials, KINTEK's precision lab equipment and consumables are designed to optimize your CVD process. Our expertise ensures you achieve the perfect balance of purity, density, and uniformity for your substrates.
Contact our experts today to discuss how KINTEK can support your laboratory's coating challenges and drive your innovation forward.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- What are the process capabilities of ICPCVD systems? Achieve Low-Damage Film Deposition at Ultra-Low Temperatures
- What is the difference between PECVD and APCVD? Choose the Right CVD Method for Your Application



















